 |
 |
Imagine this: A 19-year-old female, an extended-wear contact lens patient, presents with a red painful eye that has mucopurulent discharge. She has a large epithelial defect, stromal infiltration and a heavy anterior chamber reaction. Her corrected visual acuity is 20/200.
Given her contact lens wearing history and clinical appearance, you suspect bacterial keratitis. You prescribe a fourth-generation fluoroquinolone hourly and a cycloplegic agent, and ask her to return in 24 hours for follow-up.
At next-day follow-up, there is no longer any mucopurulent discharge, and the patient says she feels slightly better. But, there is still significant injection, and the anterior chamber reaction has slightly worsened with the advent of
hypopyon.
The ulcer has not changed in size, shape or depth, which at this point is a good sign that the antibiotic is effective.
To address the inflammation and reduce corneal scarring, you prescribe a topical steroid to be used concomitantly with the antibiotics and cycloplegic. Again, you schedule her to return in 24 hours.
The next day, the patient says she feels much better. The anterior chamber reaction and conjunctival injection are reduced, and the ulcer has begun to re-epithelialize.
As a result, all medications are continued unchanged.
Pharmacologic Misadventure
During the next three days, the patient continues to improve, and her eye appears white and quiet. However, the ulcer fails to completely re-epithelialize.
In most cases of ocular infection, the invading organism is rapidly eradicated, especially given the clinical efficacy of the fourth-generation fluoroquinolones.
At this point, however, you may erroneously believe that the antibiotic, for some reason, is not totally effective and decide to switch to or add another topical antibiotic.
Thus begins what we call a pharmacologic misadventure. This
misadventure involves a failure to recognize the crucial point when the therapy becomes more damaging than the disease.
This failure can then result in iatrogenic disease and failure to healthis months topics.
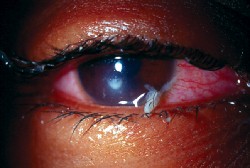 |
| The above photo shows the eye of the actual patient described here in the column. Notice the culture-proven Pseudomonas keratitis that has mucopurulent discharge. |
Iatrogenic Disease
Iatrogenic ocular surface disease is an overlooked cause of treatment failure, mostly because it closely resembles other causes of surface disease.1 One epidemiological study, in a tertiary setting, identified 13% of keratoconjunctivitis cases as iatrogenic. Healing was prolonged, taking seven to 93 (median 28.5) days.1
Another report described 19 patients who had toxic ulcerative keratopathy. These patients had been diagnosed with other conditions for which they were overtreated.2 Many of the patients with iatrogenically induced toxic keratopathy had significant ocular surface disease, such as keratoconjunctivitis sicca, previous intraocular surgery, herpes simplex infection and herpes zoster infection.
Many topical medications can affect corneal healing.3-7 In some cases, the medication itself is responsi-ble.3,4 In other cases, the preservatives in the medica- tions affect the ocular surface.4,5 There is compelling
evidence that preservative-free preparations are associated with significantly less ocular toxicity.4,5,8
One study suggests that moxifloxacin (Vigamox, Alcon) induces less damage to the corneal epithelium than other antibiotic solutions, perhaps because it is self-preserved and has no additional preservatives.4
Management of patients who have iatrogenic complications involves discontinuation of the offending agent, if possible, or switching to a less caustic substitute.1,2 Preservative-free therapies, as discussed above, should be considered whenever possible.
Treatment
So how should you proceed with the above bacterial keratitis patient who has not completely healed?
We strongly recommend that you discontinue therapy when all signs of infection have been suppressed. Then, use a non-preserved artificial tear, such as Refresh Celluvisc (Allergan), and allow the cornea to heal on its own.
Recently, we have been successful with Systane (Alcon). It provides long-term protection of the cornea against desiccation, and has no apparent deleterious effects on epithelial cells. Systane creates an environment in which damaged corneal epithelium can recover normal barrier function. The combination of ingredients in the formulation appears to provide an effective mucomimetic artificial tear product.9
The above case and how this patient was handled were both real. After 24 hours of preservative-free tearsSystane, following abrupt cessation of the initial therapeutic medicationsher epithelium completely healed.
We must not forget that prolonged treatment can cause problems for our patients, and that sometimes the best therapy is no therapy at all. On a final note, we must also not underestimate the therapeutic ability of artificial tears.
Both Drs. Sowka and Kabat acknowledge they are speakers for Alcon.
1. Dart J. Corneal toxicity: the epithelium and stroma in iatrogenic and factitious disease. Eye 2003 Nov;17(8):886-92.
2. Schwab IR, Abbott RL. Toxic ulcerative keratopathy. An unrecognized problem. Ophthalmology 1989 Aug;96(8):1187-93.
3. Liu GS, Trope GE, Basu PK. Beta adrenoceptors and regenerating corneal epithelium. J Ocul Pharmacol 1990 Summer;6(2):101-12.
4. Kovoor TA, Kim AS, McCulley JP, et al. Evaluation of the corneal effects of topical ophthalmic fluoroquinolones using in vivo confocal microscopy. Eye Contact Lens 2004 Apr;30(2):90-4.
5. Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol 2002 Apr; 86(4):418-23.
6. Flach AJ. Corneal melts associated with topically applied nonsteroidal anti-inflammatory drugs. Trans Am Ophthalmol Soc 2001;99:205-12.
7. Lin CP, Boehnke M. Effect of fortified antibiotic solutions on corneal epithelial wound healing. Cornea 2000 Mar;19(2):204-6.
8 Laflamme MY, Swieca R. A comparative study of two preservative-free tear substitutes in the management of severe dry eye. Can J Ophthalmol 1988 Jun;23(4):174-6.
9. Ubels JL, Clousing DP, Van Haitsma TA, et al. Pre-clinical investigation of the efficacy of an artificial tear solution containing hydroxypropyl-guar as a gelling agent. Curr Eye Res 2004 Jun;28(6):437-44.

