 |
Caring for patients with dry eye disease (DED) has always been a complex process that begins with a search for the underlying etiologies at play. Whether a patient has aqueous-deficient dry eye (ADDE) or evaporative dry eye (EDE) is a crucial distinction for management purposes, yet they are not mutually exclusive. Research now suggests 30% to 70% of dry eye patients may have a hybrid of both forms.1 This article explores the new understanding of mixed dry eye presented by the Tear Film and Ocular Surface Society’s (TFOS) Dry Eye Workshop (DEWS) II, and how to manage it in your clinic.
 |
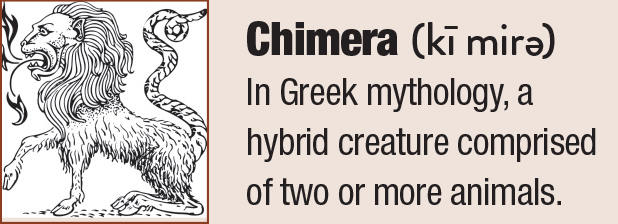
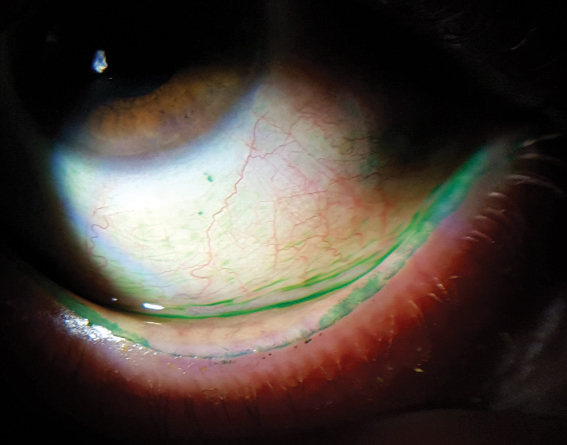
| This lower lid shows signs of obstructed meibomian glands, and digital pressure releases thickened and cloudy meibum. Click image to enlarge. |
Update Your Outlook
The TFOS DEWS II revised definition characterizes DED as a loss of tear film homeostasis accompanied by ocular symptoms. Tear film instability and hyperosmolarity, ocular surface inflammation and damage and neurosensory abnormalities are all included as possible etiologies.2
Additionally, the revised classification system now indicates that ADDE and EDE are no longer two separate entities; rather, they are coexistent on a continuum.2 Considering epidemiological and clinical studies suggest that most DED is evaporative in nature, the report shows the majority of this continuum is EDE.2,3 According to DEWS II, tear hyperosmolarity and instability are the two major players of DED. Tear hyperosmolarity—the increased osmolarity of the tears compared with the epithelial cells—leads to reduced epithelial cell volume and increased solute concentrations.4 In ADDE, hyperosmolarity is created by a reduction of lacrimal secretion. Reduced tear secretion caused by lacrimal gland dysfunction in age-related DED is just one example of ADDE. In EDE, however, the hyperosmolarity is created by an excessive evaporation from the tear film.2 The tear film lipid deficiency from meibomian gland dysfunction (MGD) is a common example of the excessive evaporation seen in EDE. The DEWS II report recognizes that these two subtypes, ADDE and EDE, can often coexist as a hybrid or mixed dry eye.
 |
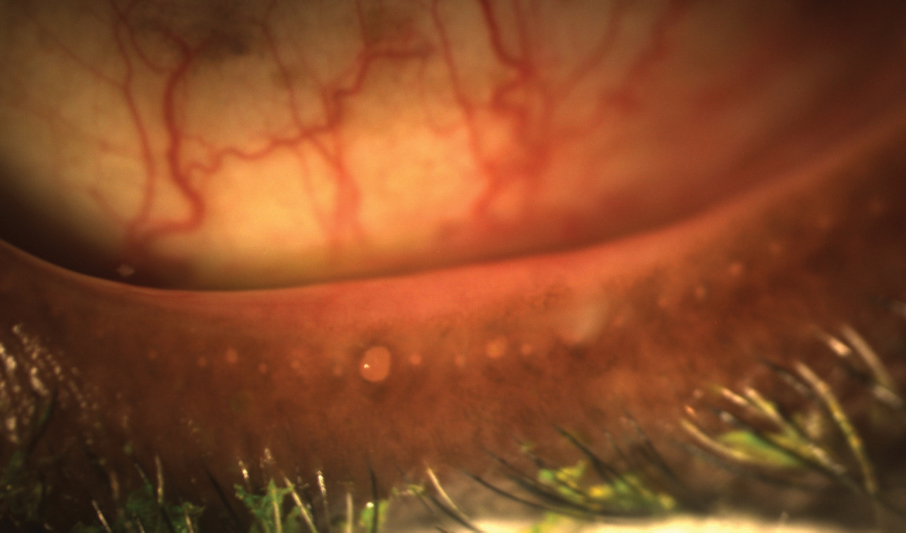
| Ocular surface staining, as seen here, can help to reveal dry eye. |
Diagnostic Maze
When diagnosing the primary etiology of a patient’s dry eye, an important first step is determining if it’s due to a lipid or an aqueous deficiency. Unfortunately, because the two are closely related, each able to affect the other, getting to the bottom of the problem isn’t as easy as it sounds. For one, researchers hypothesize that the early and late stages of DED may differ in their clinical features.5 DED may initially present as purely ADDE or EDE, but as it progresses, the characteristics of both will likely become more pronounced, presenting as a mixed form of DED.3,5 Thus, a patient with mild ADDE may present with low aqueous tear secretion secondary to lacrimal dysfunction and a normal evaporative rate in the absence of an eyelid or ocular surface-related cause. If the patient’s DED progresses to a more severe form, it may become associated with a slow tear film lipid layer spreading, resulting in an increase in tear evaporation—a hybrid of ADDE and EDE.5
To understand mixed dry eye, clinicians must first understand the many physiological changes at play.
ADDE. This is composed of two subcategories: Sjögren’s syndrome (SS) dry eye and non-SS dry eye. SS is an autoimmune disorder consisting of immune cell infiltration of exocrine glands (i.e., salivary and lacrimal) and systemic complications secondary to events such as autoantibody production and lymphocytic infiltration of organs.6 In SS dry eye, immune cell infiltration of lacrimal and salivary glands leads to glandular destruction and sicca symptoms.6 This destruction and additional inflammatory changes lead to a loss of aqueous tear flow.6
The clinical features of non-SS dry eye, although similar to SS dry eye, occur later in life and are usually less severe.6 Apart from the glandular destruction or dysfunction, reduction in lacrimal tear secretion may be attributed to a decreased corneal sensitivity to all sensory modalities. Age-related ADDE is the most common form of non-SS dry eye, and its incidence increases around age 50.6 With this form, the accumulated changes in the ocular structures and function over time may contribute to the development of dry eye. Research has proposed many mechanisms, including decreased corneal sensitivity to mechanical and chemical stimuli with age and lacrimal gland damage from oxidative stress.6 Congenital and acquired etiologies that may contribute to non-SS include congenital alacrima, sarcoidosis, viral infection, radiation injury, Stevens-Johnson syndrome, mucous membrane pemphigoid, topical anesthetic use, refractive surgery, contact lens wear, neurotrophic keratitis and diabetes mellitus, to name a few.6
EDE. Tear hyperosmolarity is an integral component of DED, and some consider all forms of DED to be evaporative, since tear hyperosmolarity cannot occur without evaporation.6,7 EDE, then, is a hyper-evaporative state resulting from the loss of an evaporative barrier function of the tears or reduced surface wettability.6 Like ADDE, EDE is subdivided into two categories: lid-related and ocular-surface related. The most common cause of EDE is MGD, specifically obstructive MGD.7,8 This causes low meibum delivery and is either caused by a displacement of the terminal ducts or secondary to a hyperkeratinization process to the terminal ducts.
Meibomian glands are large sebaceous glands vertically located in the tarsal plate and open onto the eyelid margin just anterior to the mucocutaneous junction. In cicatricial MGD, the terminal duct is dragged posteriorly into either the marginal conjunctival or tarsal mucosa.6,9,10 In non-cicatricial MGD, terminal ductal keratinization occurs, and sloughing of the ductal cells into the lumen forms keratotic plugs.6,7 The meibum thickens and becomes cloudy, blocking the ducts and orifices, eventually leading to secondary disuse or pressure atrophy of the glands.6 In addition, the tear film’s lipid content is reduced secondary to the absence of normal meibum, promoting entry into the vicious circle of DED.8
As DED progresses and the ocular surface is exposed to desiccating stress, a compensatory secretory tear response, driven by impulses from corneal cold-modality thermoreceptors, slows disease progression by dampening the rise in tear osmolarity. This is triggered by both tear hyperosmolarity and surface cooling. Not only do these cold modality fibers increase basal tearing, they lead to an increased blink rate, which refreshes the tear film more often and helps to decrease the tear osmolarity.3,6 This compensatory response leads to eye awareness, contributes to discomfort symptoms and may explain why some patients complain of epiphora in MGD-related DED.6 In pure ADDE (no signs of EDE such as MGD) and pure EDE (abnormal tear film lipid layer in the presence of normal lacrimal function), the compensatory tear response helps to slow the disease progression.5
However, as the disease worsens, it may also include a progressive loss of corneal sensitivity and the corresponding compensatory response, further increasing disease severity. In worsening ADDE, the rate of the tear film lipid layer spreading diminishes progressively and is amplified when the compensatory reflex tearing is reduced. One study suggests this reduced tear film lipid layer spreading is likely accompanied by an increase in evaporative loss, converting the ADDE into mixed dry eye. In EDE, corneal damage and a loss of reflex sensory drive to the lacrimal gland will result in further tear hyperosmolarity and evolve the pure EDE to a mixed dry eye.5
Circling the TargetThe DEWS II concept of the vicious circle of dry eye is that of a self-perpetuated, cyclical system with multiple entry points, including tear hyperosmolarity, tear film instability, apoptosis and inflammation—all of which are related to each other. Tear film instability caused by a rapid tear break up leads to surface drying and hyperosmolarity of the epithelium.8 Tear hyperosmolarity stimulates a cascade of signaling events within the ocular surface epithelial cells that generates the release of inflammatory mediators, such as inflammatory cytokines and proteases. These agents recruit inflammatory cells to the surface, exponentially increasing inflammatory mediators. This leads to decreased expression of glycocalyx mucins and to the apoptotic and non-apoptotic death of epithelial cells and the loss of goblet cells. The chain of events leads to ocular surface damage and destroys the ocular surface’s defense system. This heightens the ocular surface hyperosmolarity, continuing the vicious circle.6 Extrinsic factors such as ocular surface inflammation from allergic eye disease, topical preservative toxicity, topical anesthesia, corneal surgery, contact lens wear, systemic medications such as antihistamines and beta-blockers and environmental elements such as low humidity, high airflow and high temperature can also disrupt reflex tear secretion or increase tear film instability and initiate entry into the vicious circle.6,8 Understanding this vicious circle is crucial for selecting appropriate treatment and therapies to disrupt it.8 Of course, once initiated, the vicious circle may continue despite the reduction or elimination of the initial cause, and treatment may need to target multiple mechanisms at play. Patients within the vicious circle of DED suffer from both visual and ocular discomfort arising from tear film instability, tear hyperosmolarity, inflammatory mediators, hypersensitivity of corneal nerves due to the loss of the epithelial barrier and friction between the globe and lids. A loss of lubrication due to reduced tear volume, loss of goblet cell mucin, degradation of glycocalyx mucin and loss of lubricin may cause damage to orbital structures, as is seen in lid-parallel conjunctival folds, lid wiper epitheliopathy and superior limbic keratoconjunctivitis.6 |
Tools of the Trade
A battery of diagnostic tests can help confirm DED and whether ADDE or EDE is the predominant cause of a patient’s mixed dry eye.
The TFOS DEWS II DED management algorithm starts with triaging questions to help exclude conditions that may mimic DED (such as allergic eye disease) and assess risk factors the patient may have such as office environment, contact lens wear, systemic history and medications and topical medications.11 Various questionnaires, such as the Ocular Surface Disease Index and Dry Eye Questionnaire are useful for gauging the patient’s visual symptoms and DED’s impact on their quality of life. These validated questionnaires also provide a means to monitor patients’ treatment progress.11
TFOS DEWS II recommends diagnostic tests that assess homeostasis markers, tear break-up time (TBUT), osmolarity and ocular surface staining (>5 corneal spots, >9 conjunctival spots, or ≥2mm and ≥25% width lid margin). TBUT is commonly performed with fluorescein, although non-invasive TBUT has become increasingly popular since fluorescein may reduce tear film stability.
When measuring osmolarity with a TearLab osmometer or OcuSoft i-Pen, ≥308mOsm/L in one eye or an inter-ocular difference >8mOsm/L is considered a positive result.
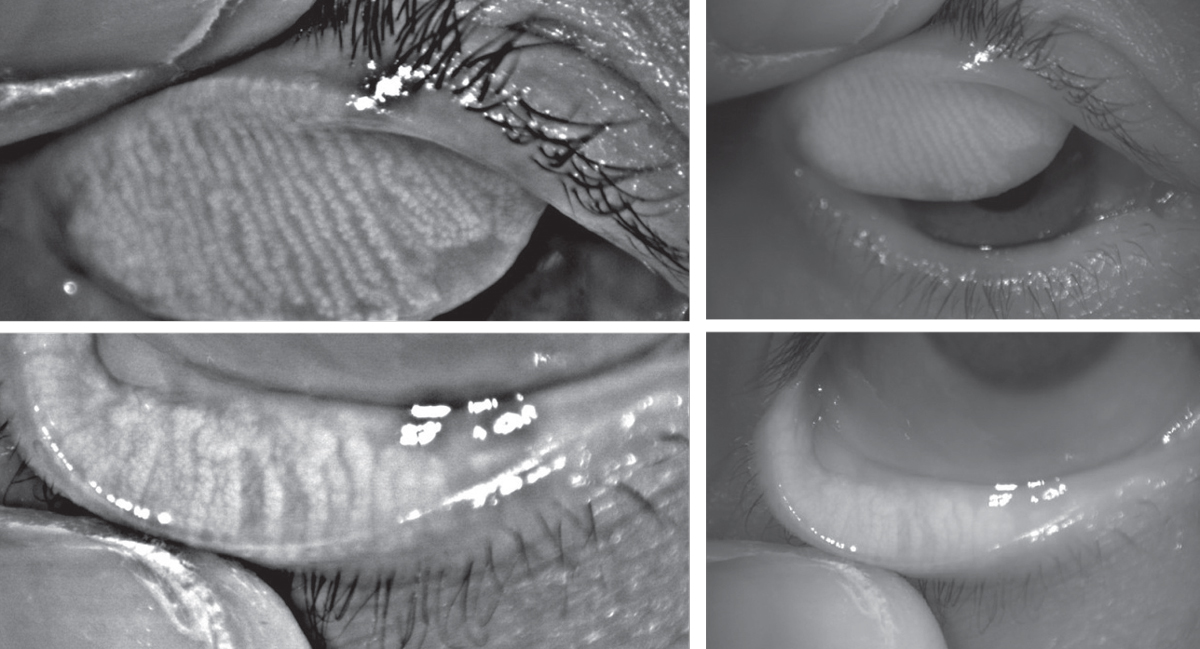 |
| Above, infrared images of the upper and lower eyelids with non-invasive meibography shows healthy, long meibomian glands. In comparison, the patient below has MGD. The glands are greatly shortened compared to those above, and the areas devoid of glands indicate dropout. Click images to enlarge. |
 |
Clinicians should also assess for ocular surface staining with fluorescein, lissamine green or both. Corneal staining appears in the later stages of DED, and the combination of fluorescein and lissamine green staining is recommended as the diagnostic technique to evaluate for ocular surface damage.11
The diagnosis of DED is made when the patient is symptomatic and has one positive homeostasis marker. However, signs alone warrant management to prevent the manifestation of DED.
To determine if it is predominantly EDE or ADDE, clinicians should evaluate the tear volume, lipid thickness and dynamics and posterior lid. For tear volume measurement, one can assess the tear meniscus at the slit lamp or with meniscometry or tests such as phenol red thread test and Schirmer test. The Schirmer test without anesthetic is recommended for confirmed severe ADDE.
Meibography allows observation of the silhouette of the meibomian glands and clinicians can observe structural changes such as gland shortening. To evaluate meibomian gland function, the clinician must check the expressibility of the glands with digital pressure and the meibum quantity and quality. Normal eyelids will have clear meibum easily expressed with gentle pressure; in MGD, the meibum loses clarity and is more difficult to express. Lipid thickness can be assessed with interferometry, and clinicians should also determine if the patient’s blink is complete. These tests should be performed in the order of least to most invasive, as the testing sequence can affect results (Table 1).11
Although these tests can help the clinician determine if the DED is primarily caused by aqueous deficiency or evaporation, DED often manifests as a hybrid, and treatment will need to target the primary cause as well as any other etiologies involved.
Table 1. Dry Eye Diagnostic Testing11 | |
| Tear film stability | Tear break-up time
|
| Tear volume/production |
|
| Tear film osmolarity |
|
| Ocular surface |
|
| Posterior eyelid |
|
| Anatomical abnormalities |
|
Therapy Options
The goal of DED management is to break the vicious circle, restore homeostasis of the ocular surface and prevent a return to the vicious circle—not always an easy task.1 DED therapy is often complicated and, at times, difficult because of its multifactorial nature and the timeline required for treatment. In addition, each patient’s dry eye management regimen will vary depending on the underlying etiologies, disease severity and any contributing extrinsic factors such as environment or medications. Patients may suffer from psychological factors such as depression and stress because of their dryness and are often seeking a “quick fix.”1 A clinician’s first job after diagnosing DED and differentiating between ADDE, EDE or mixed dry eye is properly educating the patient on the condition, treatment expectations and timeline.
Typically, management begins with low-risk and commonly available therapies such as over-the-counter lubricants for early stage disease; therapies advance as disease severity increases (Table 2).1 Follow-up is essential to check for improvement in symptoms and signs and to ensure the patient is compliant. If the patient’s signs and symptoms do not improve within three months, clinicians should explore other management options. For patients who are not responding to treatment, a more advanced treatment or consideration of other causes may be necessary.
ADDE. Treatments for this form of DED include tear replacement with ocular lubricants or artificial tears. Typically, artificial tears are recommended for three to four daily applications and may be low-viscosity solutions. As disease severity increases, non-preservative solutions and lubricants that have a higher retention rate such as gels or ointments may be necessary. Punctal occlusion may help with tear conservation and should be considered for dry eye secondary to refractive surgery, symptomatic contact lens wear, ADDE secondary to systemic diseases and dry eye with a rapid TBUT. However, it is controversial to use punctal plugs if the patient has an inflammatory component, as it may prolong the presence of pro-inflammatory cytokines on the ocular surface.1 Moisture chamber goggles and humidifiers may help to slow tear evaporation, especially if the patient lives or works in an adverse environment. For patients with severe ADDE or those not responding to other treatments, consider autologous or allogeneic serums. Oral secretagogues may be considered for ADDE or mixed dry eye patients with SS in particular.1
 |
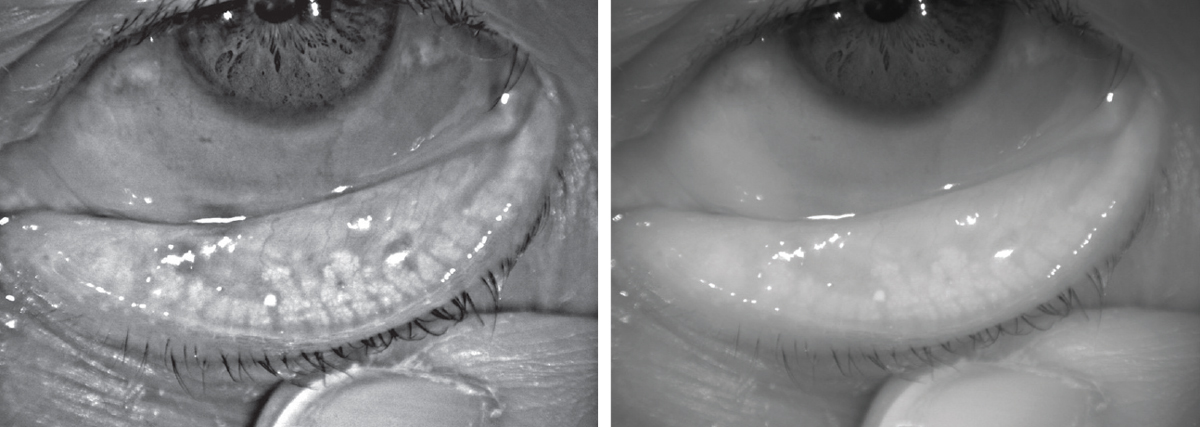
| Lissamine green staining on a patient’s conjunctiva and lid margin. |
EDE. Treatments for this form or lid abnormalities include lid hygiene with diluted baby shampoo or commercially available lid cleansers and warm compresses with a moist, heated cloth, masks or infrared warm compression devices. If ocular lubricants are warranted, clinicians may want to recommend lubricants containing lipids.
Physical meibomian gland treatments with warm compresses, gland expression or intraductal probing help improve or restore function by removing ductal obstruction. Intraductal probing should be reserved for MGD patients unresponsive to conventional treatment because it is invasive and comes with a risk of damage to the ductal system.
A short course of topical antibiotics such as ofloxacin may help reduce the bacterial load on the lid margin (typically in patients with DED associated with blepharitis).1 Doxycycline and minocycline are used for their anti-inflammatory and lipid-regulating roles and may be dosed at 50mg to 100mg once or twice a day for a short duration or low dose (20mg) on a long-term basis. Another antibiotic, azithromycin, is a good option for patients with MGD and rosacea; its anti-inflammatory properties may also help control lid inflammation and lid bacterial flora.
Anti-inflammatory therapies for DED include limited-duration corticosteroids. Long-term use of corticosteroids may be used for severe DED or non-responsive patients, but risks (ocular hypertension, cataracts and opportunistic infections) exist. For these patients, repeated short-term pulse therapy may be a better option. Other commercially available anti-inflammatory products include Restasis (cyclosporine, Allergan) and Xiidra (lifitegrast, Shire).1
Other treatment options include contact lenses and amniotic membranes. Patients may also benefit from dietary or environmental modifications such as increased water-intake, omega-3 fatty acid supplementation, altering systemic medications such as beta-blockers, antihistamines and antidepressants, and switching to preservative-free ocular medications. If the patient has chronic symptoms but limited clinical signs and lack of response to treatment, then clinicians may need to consider a non-DED etiology such as neuropathic pain.1,11
As a multifactorial disease that can present with a mixture of aqueous-deficient and evaporative dry eye etiologies, DED requires a treatment that targets the various causes of the dry eye and breaks the vicious circle. DED is progressive and if not treated early, may become severe in nature and cause the patient significant discomfort. Patients who are not responsive to conventional therapies may benefit from a referral to a specialty dry eye clinic that has advanced management options.
Table 2. DED Management and Treatment Recommendations1 |
Step 1
|
Step 2
|
Step 3
|
Step 4
|
Dr. Kwak is an Illinois College of Optometry graduate and did her Primary Care residency at Salus University Pennsylvania College of Optometry. After years of practice at an anterior segment practice, she recently joined Salus University as a clinical faculty member.
1. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(4):580-634. |

