 In 2008, a 64-year-old white male glaucoma patient was referred to me from a colleague in Connecticut. This doctor had treated the patient for several years and sent him to me for further care because the patient was relocating to my area.
In 2008, a 64-year-old white male glaucoma patient was referred to me from a colleague in Connecticut. This doctor had treated the patient for several years and sent him to me for further care because the patient was relocating to my area.
At that time, he was on Travatan (travoprost, Alcon) HS OU for about three years. His other medications included only aspirin (81mg daily as prophylaxis for cardiovascular disease) and generic vitamins.
He reported no family history of glaucoma, and said he’d been diagnosed with glaucoma based on elevated intraocular pressure and “some damage” to his right eye.
Diagnostic Data
At this initial visit, his best-corrected visual acuity was 20/20 OU through mild hyperopic astigmatic and presbyopic correction. Pupils were equal, round and reactive to light and accommodation with no afferent defect. Intraocular pressure measured 14mm Hg OD and 16mm Hg OS at 10:35 am.
His angles appeared to be grade 3 open OU by Van Herick estimation. His anterior segments were unremarkable and his crystalline lenses were clear.
Upon dilation, his macular examination was unremarkable, as was his vascular and peripheral retinal examination OU. Stereoscopic evaluation of his discs showed estimated cup-to-disc ratios of 0.50 x 0.60 OD and 0.40 x 0.40 OS. The right optic nerve did not respect the ISNT rule, with a somewhat thinned neuroretinal rim inferotemporally. The neuroretinal rim of the left eye appeared relatively robust. Central corneal thickness measured 532μm OD and 549μm OS.
Prior to the patient’s arrival at my office, the previous provider sent a summary of his clinical care, which included the rationale for initiating therapy: increased IOP and optic nerve asymmetry. Prior to treatment, his IOP averaged 24mm Hg OD and 21mm Hg OS. After treatment, his IOP averaged in the mid-teens OU.
Following his initial visit with me, I saw the patient on a regular basis over the next few years. During this period, his IOP remained in the mid-teens in both eyes.
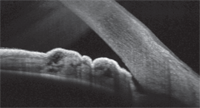
Anterior segment OCT image of the temporal angle of the left eye. Note the anterior placement of the iris adjacent to the trabecular meshwork, which was not apparent with gonioscopy. Could this be causing the patient’s recent rise in IOP?
Gonioscopy showed grade 3 open angles OU with mild trabecular pigmentation and no angle abnormalities in either eye. Heidelberg Retina Tomograph-3 (HRT-3, Heidelberg Engineering) imaging of the optic nerves correlated well with my clinical observations of his optic nerve and neuroretinal rim appearances OU. Sequential HRT-3 Topographical Change Analysis (TCA) showed stable neuroretinal rims.
Threshold standard automated perimetry demonstrated no discernable field defects OD or OS over several years, though scattered areas of depression were occasionally noted in the superior arcuate area of the right eye.
In late 2010 though, his IOP in both eyes began to creep upward, more in the right than in the left. IOP in the right eye increased to 20mm Hg on average over four visits, while IOP in the left increased to an average of 17mm Hg during the same time. Given this modest but demonstrable IOP increase, I added Azopt (brinzolamide, Alcon) BID to the right eye only.
Subsequently, IOP in the right eye decreased to 18mmHg, with lower fluctuations in IOP ranges and with no structural or functional changes observed in the right eye’s visual fields or optic nerve characteristics. All indices in the left eye remained stable.
But recently, over the past four visits, the patient has had another increase in IOP in both eyes. When seen most recently in mid-July, his IOP was 21mm Hg OD and 19mm Hg OS. While the right eye had sustained more damage initially, both optic nerves still showed no progressive structural damage, nor were they exhibiting any significant functional damage.
Evaluation and Management
Because there are no demonstrable structural or functional changes at this time, I’m going to continue to monitor the patient without alteration of his therapeutic regimen.
Discussion
When managing a glaucoma patient over a prolonged period, any of the three primary indices of stability—IOP, visual fields and optic nerve structure—might begin to change. In this patient’s case, it’s his slowly rising IOP in his right eye. This crossroads is commonly encountered in managing glaucoma patients: What should you do when you’re beginning to lose control of the disease, and when do you make that change in therapy?
In this case, let’s think about why his IOP is becoming seemingly less controllable and gradually increasing with time. In the simplest scenario, IOP increases either because of increased outflow obstruction or because of reduced efficacy of the anti-glaucoma medications—or perhaps both mechanisms at the same time. (Regarding the reduced efficacy of current medical therapy, consider that either the medications themselves are losing their effectiveness or perhaps the glaucoma is simply becoming more recalcitrant to therapy.)
In either case, once you’re certain that there has been repeatable, demonstrable change, a modification of therapy is warranted. And that may include the addition of more medications, a substitution of medications, or progression to laser or other surgical intervention. Which option you choose to regain control of the IOP will vary on a case-by-case basis.
In this patient’s case, medical management of his current glaucoma state must be considered along with periodic examination of another very important aspect of IOP control: outflow mechanisms. To do so, we need to take into account another diagnostic method of glaucoma management: serial gonioscopy.
A New Angle on Gonio
Gonioscopy gives us a great view of the outflow structures, but it can be a difficult procedure to perform in some patients (though with practice, the technique does become easier).
In addition to the problem of actually performing the procedure, the other limitation with gonioscopy is the seemingly low yield of practical information it provides, especially in patients with open angles. But gonioscopy does indeed give us valuable information about the outflow structures (even on patients with open angles): the amount of trabecular pigmentation, the presence of iris processes or peripheral anterior synechiae, and the contour of the iris plane as it relates to the angle (bowed, flat, plateau).
That said, I performed gonioscopy on this patient at least yearly since I assumed his care, but I was not able to ascertain any significant demonstrable change in his gonioscopic appearance over time. Part of the difficulty in appreciating subtle changes in the angle over time lies in the fact that we do not take serial gonioscopic photographs—in fact, I know of only a few clinicians who take such photos for documentation purposes.
However, OCT imaging of the anterior segment may give us the ability to do just this: monitor subtle gonioscopic changes over time. With the advent of anterior segment OCT imaging systems, examination of the anterior chamber angle has become somewhat easier to perform than doing gonioscopy, and the detail obtained with such imaging systems is often greater.
To that end, once I realized that this patient’s IOP was slowly increasing despite medical therapy, I recently performed OCT of his angles. His anterior segment OCT images were interesting because, while they showed open angles, a good portion of his posterior trabecular meshwork in each eye was partially covered temporally by a roll of iris tissue—something that I could not appreciate gonioscopically.
Could this iris tissue be contributing to his gradual increase in IOP by impeding his trabecular outflow? It very well might.
Does this information factor into what steps I’ll take next if I decide to modify his therapy? It most certainly will.

