He was last seen by his glaucoma doctor approximately nine months earlier and reported that he had been due visit again this past September, but was just getting around to following through with that task.
Current systemic medications included daily lisinopril for hypertension and alprazolam and loratadine as needed. He reported no allergies to medications. Ophthalmic medications included Simbrinza (brinzolamide/brimonidine tartrate ophthalmic suspension, Alcon) twice a day in both eyes and generic latanaprost at bedtime in both eyes. He reported that he has been on the same glaucoma medications for the past two to three years and, furthermore, that his glaucoma doctor was pleased with his tolerability of the medications and their efficacy.
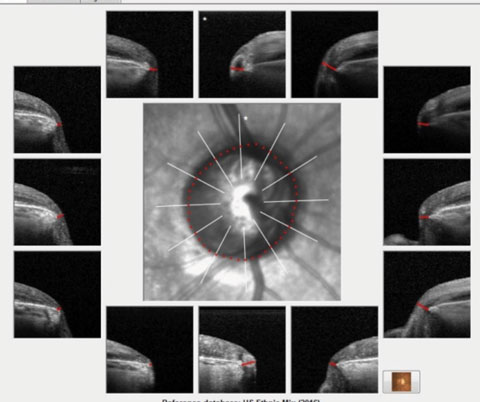 |
| Fig. 1. These images show the Bruch’s membrane opening overview of the patient. Note the thinned neuroretinal rim tissue in all sectors, especially inferotemporally. |
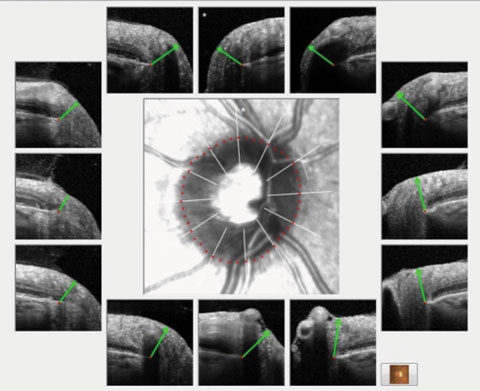 |
| Fig. 2. A non-glaucomatous eye in the context of BMO minimum rim width and nine representative radial scans of the optic nerve. Note the robust neuroretinal rim in each scan as evidenced by the green marker, as compared with Figure 1 that shows significantly eroded margins. |
Presentation
Entering visual acuities were 20/40 OD and 20/30- OS. Best-corrected visual acuities were 20/30+ OD and 20/20- OS. Pupils were reactive to light and demonstrated no afferent pupil defect by inverse Marcus Gunn testing. The right pupil in the right was surgically distorted and larger than the left, with evidence of iris sphincter damage. He subjectively reported that the vision in his right eye has been bad for some time due to the glaucoma. A quick view of the posterior pole demonstrated advanced glaucomatous optic neuropathy.A slit lamp examination of the anterior segments was remarkable for several items, including a patent trabeculectomy bleb, a surgical PI, sectoral iris atrophy and the aforementioned pupillary irregularity—all in the right eye. He was pseudophakic OU with clear posterior chamber intraocular lenses (IOLs) and clear intact posterior capsules. Applanation tensions were 14mm Hg OD and 15mm Hg OS at 11am. Pachymetry readings were 531µm OD and 544µm OS. Prior to dilation, threshold visual fields were performed with reasonable reliability indices. The fields in the right eye demonstrated a dense, superior arcuate scotoma involving fixation, with a pronounced nasal step; the left visual fields demonstrated above and below arcuate defects not involving fixation.
Through dilated pupils, the IOLs were well centered in the capsular bags. There were bilateral posterior vitreous separations. His cup-to-disc ratio in the right eye was estimated to be 0.95 x 0.95; in the left, it was 0.80 x 0.85.
The macula in the right eye was characterized by a centrally located epiretinal membrane (ERM) with epiretinal wrinkling along with fine retinal pigment epithelium (RPE) mottling present in both eyes. The retinal vascular examination was remarkable for mild arteriolarsclerotic retinopathy. The peripheral retinal examinations were remarkable only for cystoid degeneration in both eyes.
HRT 3 images were obtained, as were optical coherence tomography (OCT) optic nerve and macular scans as per our office protocol (Figures 1 and 2).
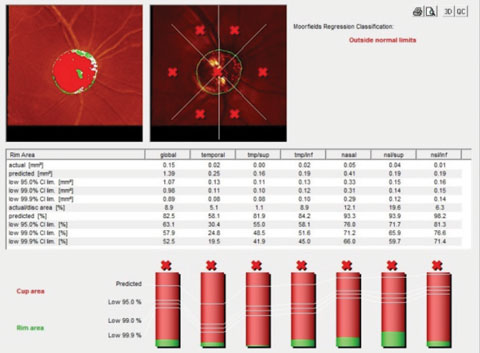 |
| Fig. 3. The HRT 3 image of the right optic nerve shows advanced disease and a thin neuroretinal rim. |
Discussion
This patient is new to the office, new to me, presenting with no previous information insofar as records, with advanced glaucoma in both eyes and decreased vision in the right that apparently is not of recent onset. One of the most important things to do at visits such as these, in my opinion, is to establish a rapport with the patient to foster a comfort zone so that they will continue their care with us and, equally important, heed my advice moving forward. Just as he is a new patient to me, I am a new doctor to him. He apparently was comfortable enough with his previous glaucoma doctor to undergo bilateral SLT procedures, as well as a trabeculectomy in his right eye and maintain that care for several years. Now that he has moved to this area, someone needs to pick up his care, and he has selected me for this job.In an initial visit, we must also establish whether the patient is stable and, if not, make the appropriate changes in therapy. Fortunately, in this case, he appeared relatively stable and I did not need to alter his therapy.
I also took the opportunity to establish several important baseline measurements of this patient, including optic nerve imaging and visual field testing. His HRT 3 images were consistent with the clinical picture of advanced disease in the right eye (Figure 3) and the left.
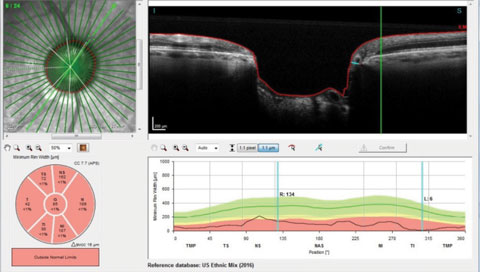 |
| Fig. 4. An overview of the Bruch’s membrane opening in a patient with advanced disease. |
Can You Picture This?
Imaging technology is rapidly evolving, and it is incumbent upon optometrists to be cognizant of these technological advances and their clinical implications. Clinicians must also be aware of the implication of using their instruments’ reference databases.This case is a good example of some imaging capabilities available to monitor optic nerve damage in glaucoma.
Figure 4 shows a radial scan through the right optic nerve, looking specifically at Bruch’s membrane opening and the minimum rim width of the ganglion cells as they pass posteriorly into the optic nerve. The reference database in the same image is that of the ethnic mix representative of the overall US population and is represented on the bottom right of the image. Clinically, we can readily see the patient has advanced disease, but interestingly enough, on the radial scan selected, the minimum rim width inferotemporally is reduced to a meager 6µm of remaining tissue, and all Garway-Heath sectors are clearly outside normal limits. For clinicians like myself who believe that damage can be detected by changes to the neuroretinal rim, such imaging technologies are appreciated.
When employing OCT scanning technologies, many use the retinal nerve fiber layer (RNFL) perioptic circle scan. In this particular patient, again, with clearly advanced disease in the right eye, the RNFL circle scans can be somewhat deceptive. Figure 5 shows one of three circle scans taken around this patient’s optic nerve; namely, a scan 4.1mm in diameter. Note that typical RNFL circle scans are 3.5mm in diameter, and with newer software, scans can be obtained with diameter of 4.1mm and 4.7mm. The key point here is that the further from the optic nerve the circle scan is obtained, the thinner, naturally, is the neuroretinal rim. Reference databases were established for each of these circle diameters.
So even though advanced disease is readily seen on the physical slit lamp evaluation of the optic nerves, sometimes imaging data can give us a false sense of security.
In Figure 5, only one Garway-Heath sector is flagged as aberrant, implying that, at least for this particular patient, damage is more readily visible in the neuroretinal rim than the RNFL. Thus, it is critical that we are aware of precisely what our technology is telling us.
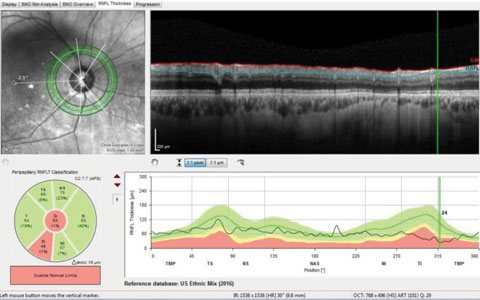 |
| Fig. 5. These circle scans measure the perioptic retinal nerve fiber layer. Highlighted in green in the upper left portion of the image is the 4.1mm scan, which shows statistical thinning only in the inferotemporal sector. |

