Although pseudoexfoliation syndrome is the most common identifiable cause of open-angle glaucoma (OAG) worldwide, the condition has many unique qualities that merit review.
For example, pseudoexfoliative glaucoma (PXG) is much more aggressive than conventional OAG––intraocular pressure (IOP) may fluctuate widely, it presents more asymmetrically and it could be less responsive to topical medical therapy. Further, PXG patients often require early detection and close, careful monitoring.
Epidemiology
Extensive population studies have shown a marked difference in pseudoexfoliation syndrome incidence based on geographic location, which is highly suggestive of a racial predilection.3 The Framingham Eye Study in Massachusetts and the Blue Mountains Eye Study in Australia, for example, showed a disease prevalence of 1.8% and 2.3%, respectively; however, some European studies report up to a 50% incidence in some Scandinavian countries.3,4 It is important to note that psuedoexfoliation is most commonly found in whites over age 50 with a slight female predilection.2,3,5
Recent studies have implicated the lysyl oxidase-like 1 (LOXL1) gene in pseudoexfoliation syndrome, and showed that individuals with two specific LOXL1 variants were more likely to develop the condition.5 LOXL1 is responsible for the biogenesis of connective tissue, so any genetic alteration could potentially result in increased production of extracellular material.5 Additionally, genetic variants of contactin-associated protein-like 2 (CNTNAP2) and clusterin (CLU) are being evaluated as potential catalysts for PXG.5
Outside of genetic predilection, one research group postulated in 1979 that sunlight exposure might contribute to the expression of psuedoexfoliation; however, this association has not been further evaluated in recent studies.3,6 Additionally, the Nurses’ Health Study and Health Professionals Follow-up Study found an increased risk of pseudoexfoliation secondary to increased caffeine intake.5
Glaucoma Risk
As previously mentioned, pseudoexfoliation is the most common identifiable risk factor associated with open-angle glaucoma.7 Histological and electron microscope studies have documented unique pathological and structural changes to the cornea, iris, trabecular meshwork and zonules in affected individuals––all of which increase glaucoma risk.1
The primary mechanism of glaucomatous damage in pseudoexfoliation syndrome is the impedance of aqueous outflow.1 The accumulation of pseudoexfoliative material in the trabecular meshwork and subsequent degenerative changes to the tissue pose the greatest resistance to proper drainage. Additionally, it has been shown that active, localized production of pseudoexfoliative material by the endothelium of Schlemm’s canal can cause lumen narrowing.1 Proliferation of psuedoexfoliative material by the corneal endothelial cells, as well as secondary migration posteriorly over the trabecular meshwork, may further lead to increased intraocular pressure. 1
The mean intraocular pressure (IOP) of patients with PXG is up to 1.7mm Hg higher than those without it.3 The Olmsted County Study––which monitored the natural course of PXG––found that 44% of patients required glaucoma medications over a 15-year period.8 Further, glaucoma patients with pseudoexfoliation have more variable IOPs, higher rates of treatment failure and faster progression of optic nerve damage.8,9
Systemic Associations
Unlike primary open-angle glaucoma (POAG), pseudoexfoliation has a systemic component. Postmortem electron microscope examination of several visceral organs showed evidence of pseudoexfoliative material, suggesting systemic involvement.1
As in the eye, pseudoexfoliation can cause ultrastructural and immunohistochemical changes in affected organ systems. This can precipitate a variety of systemic complications, including abdominal aneurysms and an increased risk of coronary artery disease.1,10,11 Additionally, secondary pathological alterations to the tunica intima can cause vascular fibrosis and elastosis, which often increase blood flow resistance.10,11 Interestingly, a 30-year epidemiological study showed no increased risk of mortality in those with PXF.12
It is also worth noting that those with pseudoexfoliation are more likely to experience sensorineural hearing loss.13 Although not completely understood, extracellular fibrils likely accumulate in the structures of the inner ear, resulting in decreased sensitivity.13
Clinical Aspects
Physiologic ocular findings of this aggressive disease may be subtle. The ocular presentation generally is bilateral, but not necessarily symmetrical. Corneal findings in pseudoexfoliation syndrome are fairly rare; however, careful examination of the endothelium may show flakes of bright white fibrillar material.14,15 These deposits often appear as irregularly spaced aggregates, reminiscent of small filaments. The flakes can vary in shape and size, and––due to convection currents of the aqueous in the anterior chamber––may evolve over time.
The corneal endothelial cells often are affected dramatically in pseudoexfoliation syndrome patients. Most individuals exhibit fewer total endothelial cells with morphological alterations. Such endothelial changes are also seen in Fuchs’ dystrophy––although they do not present in conjunction with other pseudoexfoliative changes observed in the eye.
In certain instances, these changes are accompanied by increased central corneal thickness.14 Fine measurements via specular microscopy can show a measureable decrease in the number of endothelial cells, as well as polymorphism.15 Clinically, these cell alterations may appear as a light paracentral area of pigmentation on the corneal endothelium that’s occasionally organized in the pattern of a Krukenberg spindle.
Further evaluation should include a 360° assessment of anterior chamber depth, in addition to a measurement comparison between both eyes. Although there are many direct indications of lens dislocation, one indirect sign is an alteration in chamber depth. A lens can subluxate forward, leading to a shallow anterior chamber, or may fall backward towards the vitreous, resulting in a hyper-deep chamber.16
Examine trabecular meshwork pigementation via gonioscopy, looking specifically for pigment located anterior to Schwalbe’s line.17,18 Pseudoexfoliation patients often have an abnormally high volume of angle pigmentation, even without any other syndrome findings. Pseudoexfoliation is also classically associated with pigment loss––both at the pupillary margin and around the iris sphincter.
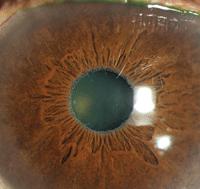
On iris transillumination, the margin may exhibit a “moth-eaten appearance.”15 Gray-white flakes may be present at the pupillary margin, and frequently are the only overt sign of disease (figure 1).15,16
| |
|
1. Telltale clinical sign of peripupillary accumulation of fibrillar material on a patient with dark irides
.
| |
|
| |
| |
|
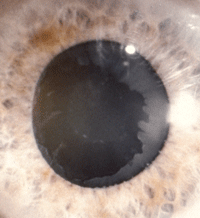
2. Apparent “bull’s eye” pattern of fibrillar material on a dilated, lightly
pigmented patient. Looking closely, there’s also a peripupillary accumulation of material.
Photo: Stephen A. Obstbaum, MD
|
Several researchers have linked deposition of this material with changes in iris function.15,16 Because the iris sphincter may be compromised, there may be a reduced response to mydriatics. Iris flutter is another indirect sign of weak zonules, which hold the lens in place; however, sometimes the iris may be more rigid in pseudoexfoliation patients, so this clinical sign may not be evident.15,16
Iris changes in psuedoexfoliation syndrome closely correlate with lenticular findings. Most often, exfoliative material presents on the anterior capsule of the lens. This finding commonly manifests in a “target” or “bull’s eye” pattern, which consists of three zones after dilation: the central disk (approximately the diameter of the pupil), the clear zone and a peripheral band of fibrillar material (figure 2).14,15 This is another sign that classically presents asymmetrically.
A definitive indication of zonular damage is phacodonesis, or lens flutter.15,16 Although crude, jolting the slit lamp table during lens examination can produce a subtle vibration or movement indicative of zonular weakness. A loose or subluxated lens is best viewed after dilation––although looking for a decentered fetal nucleus may be helpful in poorly dilating eyes.14
Individuals with pseudoexfoliative glaucoma exhibit a similar optic nerve appearance to that observed in POAG patients, including axonal loss, increased central cupping and disc rim thinning at both the superior and inferior poles.19 In advanced cases, a Drance hemorrhage may be seen.
As with other glaucomatous presentations, optical coherence tomography or Heidelberg Retinal Tomography (Heidelberg Engineering) will help you assess retinal nerve fiber layer thinning. The standard of care for visual field evaluation in all glaucoma patients is a Humphrey 24-2, which remains true for patients suspected of, or already diagnosed with, PXG.
Although the general appearance of a nerve with PXG is similar to one with open-angle glaucoma, asymmetrical presentation often helps you differentiate accurately. Interestingly, recent studies have indicated that, similar characteristics aside, nerves with pseudoexfoliation are histopathologically unique––with higher capillary density, despite axonal loss.20
Because pseudoexfoliative material can potentially rest in a multitude of anatomical locations, intraocular pressure may vary widely. A patient’s diurnal and daily IOPs can spike dramatically. Further, depending on the extent of pseudoexfoliative asymmetry, IOP varies significantly between eyes. This can make PXG treatment difficult, because establishing the patient’s baseline pressure can be a challenge. Multiple IOP measurements over a series of visits may be necessary to determine an acceptable pressure range.
There is no specific algorithm for quantifying IOP oscilation––although one report indicated that patients with PXG fluctuate the highest outside office hours, making diurnal mapping even more difficult.21 In some studies, patients’ IOPs were measured six times over a 24-hour period, which is impractical and unrealistic.22 From experience, however, it seems that a minimum of two measurements per day (i.e., one in the morning, and one in the afternoon) is both adequate and relatively easy to obtain.
Topical Hypotensive Treatment
Managing cases of pseudoexfoliative glaucoma with topical hypotensive therapy may require increased vigilance and a certain element of creativity. In 2013, Murray Fingeret, OD, indicated that 16% of PXG patients require therapeutic intervention upon initial presentation.17 So, a treatment decision may need to be made early or even immediately in the management process.
Traditional medications are less effective for PXG than POAG, but are still often used as first-line therapy because they are more effective than carbonic anhydrase inhibitors or alpha agonists.23 Both beta blockers and prostaglandin analogs are ideal places to start, with carbonic anhydrase inhibitors and alpha adrenergic agonists following as likely adjunctive medications.
Similar to the management of pigmentary glaucoma, the use of miotics has some theoretical advantage for PXG patients. Miotic agents forcefully open the trabecular meshwork and increase drainage, as well as limit pupillary movement.18,24 By reducing pupil motion, less pigment and pseudoexfoliative material are released from their respective locations.18 Take note that miotics should be used with discretion in individuals with cardiac risk factors, and may precipitate retinal detachments in some patients.25
Typically, the managing clinician will begin dosing with one class of ocular hypotensive agents, and then add secondary medications as needed. Because PXG has a higher incidence of progression and is more likely to be resistant to medical management than POAG, further therapeutic intervention often is required.26
Surgical Intervention
The Early Manifest Glaucoma Trial showed us that PXG is more rapidly progressive and is more likely to require surgical intervention than POAG.27 When topical treatment fails, or if maximum medical therapy is inadequate in achieving the desired IOP, surgical management should be considered.
• Non-incisional. Laser trabeculoplasty is a commonly performed procedure that increases aqueous outflow in eyes with POAG.28 Argon laser trabeculoplasty (ALT) was once thought to yield trabecular meshwork contraction and subsequent aqueous collector channel widening.
However, a histological study published in 2007 indicated that ALT’s effect actually causes a remodeling of the juxtacanalicular region of the meshwork.29,30 Selective laser trabeculoplasty (SLT), on the other hand, uses a Q-switched frequency-doubled Nd:YAG laser to selectively target pigmented cells within the trabecular meshwork and increase aqueous outflow.31
Use of either ALT or SLT on eyes with POAG or PXG has been found to yield comparable levels of IOP reduction across the board.28 In a series of studies, POAG eyes exhibited nearly a 22% IOP reduction one day following surgery, compared to a 29% IOP reduction in PXG eyes.28 However, at three-year follow-up, POAG eyes maintained an average IOP reduction of 34%, whereas PXG eyes remained just 21% below baseline.28 Because PXG tends to be more aggressive than POAG, pseudoexfoliative patients often require additional management once the pressure-lowering effect from ALT or SLT subsides.
• Cataract removal. It is well documented that oxidative stress in pseudoexfoliation syndrome leads to early cataract formation.32 In many cases, patients with PXG will require cataract surgery. A retrospective study of more than 1,000 patients with pseudoexfoliation showed a significant improvement in IOP after uncomplicated phacoemulsification.33 Those with PXG had a more significant and sustained IOP reduction than patients without PXG.33 Further, only about 3% of eyes with early evidence of pseudoexfoliation syndrome progressed to PXG over a 10-year postoperative period.33
• Trabeculectomy. In cases of more advanced glaucoma, trabeculectomy is one of the most commonly indicated surgical interventions.34 The procedure involves scleral flap formation and ostium creation, which allows aqueous dissipation into the subconjunctival space.34-36
Trabeculectomy for PXG patients is a successful means of reducing intraocular pressure; however, some clinicians believe there might be a higher incidence of postoperative pressure spikes compared to POAG patients who undergo the procedure.36 Combined cataract removal and trabeculectomy often yields fewer pressure spikes than trabeculectomy alone.35,37
• Minimally invasive glaucoma surgery (MIGS). These surgical procedures are relatively new, and are intended to increase aqueous drainage through the trabecular meshwork.38-40 The two most common MIGS procedures are the Trabectome (NeoMedix) and the iStent (Glaukos). Take note that only the Trabectome has been studied in a large pseudoexfoliation study sample.40
In a Trabectome procedure, the surgeon removes the trabecular meshwork through a clear corneal approach, which exposes the collector channels of Schlemm’s canal. In the study mentioned above, 173 PXG eyes underwent Trabectome surgery.40 Of these, 34% were phakic, 25% were pseudophakic and 41% underwent combined cataract and Trabectome surgery.40 At one-year follow-up, the entire patient population exhibited a mean IOP reduction of 30%.40
Although not thoroughly studied in the pseudoexfoliation population, the iStent is placed into the angle via a clear corneal incision.38 The stent is a 1.0mm nonferromagnetic, L-shaped device that is inserted into Schlemm’s canal.38 Because the iStent facilitates bypass of the trabecular meshwork, the resultant IOP is based on the circumferential flow of Schlemm’s canal, the collector ducts and the episcleral venous pressure.38 Following the procedure, IOPs typically range in the high teens.38
• Endocyclophotocoagulation (ECP). Cyclodestruction of the ciliary body as a treatment for glaucoma was first attempted in the 1970s.41 Historically, the procedure was performed transsclerally with cryotherapy, an Nd:YAG laser or a diode laser, and was reserved for eyes with severe disease and/or little potential for visual gain.41 Because the target is not visible to the surgeon transsclerally, there is risk of adjacent tissue damage and resultant inflammation, decreased visual acuity, hypotony and phthisis.42
ECP is performed intraocularly with the aid of an endoscope (usually in conjunction with cataract extraction), which permits direct visualization of the targeted ciliary body tissue.41 In a case series of 68 patients (including those with PXG), researchers documented a 34% mean IOP reduction following ECP.41 Although a large prospective study of ECP in eyes with pseudoexfoliation has not been performed, this seems like a promising therapy going forward.
Pseudoexfoliative glaucoma, a well-known member of the OAG family, is a fairly unique clinical entity. While PXG’s signs can be subtle, its impact can be visually devastating––so we are tasked with identifying the condition as early as possible.
In comparison to those with primary open-angle glaucoma, PXG patients often need topical ocular hypotensive agents earlier in the disease process. Additionally, they require more frequent follow-ups and testing, as well as adjunctive therapy, to reduce the likelihood of irreversible vision loss.
Dr. Fabrykowski is on staff at the Manhattan Eye, Ear and Throat Hospital Faculty Ophthalmology Practice, operated by Lenox Hill Hospital, in New York.
Dr. Laul is an instructor of ophthalmology at the Wilmer Eye Institute, Johns Hopkins School of Medicine, in Baltimore.
They thank Harry A. Quigley, MD, professor of ophthalmology at Wilmer Eye Institute, and Bradford J. Shingleton, MD, Ophthalmic Consultants of Boston and associate clinical professor of ophthalmology at Harvard School of Medicine for their research guidance.
1. Naumann GO, Schlötzer-Schrehardt U, Küchle M. Pseudoexfoliation syndrome for the comprehensive ophthalmologist. Intraocular and systemic manifestations. Ophthalmology. 1998 Jun;105(6):951-68.
2. Cashwell LF, Shields MB. Exfoliation syndrome. Prevalence in a southeastern United States population. Arch Ophthalmol. 1988 Mar;106(3):335-6.
3. Mitchell P, Wang JJ, Hourihan F. The relationship between glaucoma and pseudoexfoliation: the Blue Mountains Eye Study. Arch Ophthalmol. 1999 Oct;117(10):1319-24.
4. Hiller R, Sperduto RD. Pseudoexfoliation, intraocular pressure, and senile lens changes in a population-based survey. Arch Ophthalmol. 1982 Jul;100(7):1080-2.
5. Sein J, Galor A, Sheth A, et al. Exfoliation syndrome: new genetic and pathophysiologic insights. Curr Opin Ophthalmol. 2013 Mar;24(2):167-74.
6. Taylor HR. Pseudoexfoliation, an environmental disease? Trans Ophthalmol Soc UK. 1979 Jul;99(2):302-7.
7. Ritch R. Exfoliation syndrome-the most common identifiable cause of open-angle glaucoma. J Glaucoma. 1994 Summer;3(2):176-7.
8. Jeng SM, Karger RA, Hodge DO, et al. The risk of glaucoma in pseudoexfoliation syndrome. J Glaucoma. 2007 Jan;16(1):117-21.
9. Leske MC, Connell AM, Wu SY, et al. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol. 1995 Jul;113(7):918-24.
10. Schumacher S, Schlötzer-Schrehardt U, Martus P, et al. Pseudoexfoliation syndrome and aneurysms of the abdominal aorta. Lancet. 2001 Feb 3;357(9253):359-60.
11. Citirik M, Acaroglu G, Batman C, et al. A possible link between the pseudoexfoliation syndrome and coronary artery disease. Eye (Lond). 2007 Jan;21(1):11-5.
12. Svensson R, Ekström C. Pseudoexfoliation and mortality: a population-based 30-year follow-up study. Acta Ophthalmol. 2014 Mar 26. [Epub ahead of print]
13. Cahill M, Early A, Stack S, et al. Pseudoexfoliation and sensorineural hearing loss. Eye (Lond). 2002 May;16(3):261-6.
14. Crista AR. Pseudoexfoliation syndrome and cataract surgery in pseudoexfoliation syndrome. Clinic of Ophthalmology: Timisoara Medical Journal. Available at: www.tmj.ro/article.php?art=237564682127339. Accessed June 10, 2014.
15. Drolsum L, Ringvold A, Nicolaissen B. Cataract and glaucoma surgery in pseudoexfoliation syndrome: A review. Acta Ophthalmol Scand. 2007 Dec;85(8):810-21.
16. Shingleton BJ, Crandall AS, Ahmed II. Pseudoexfoliation and the cataract surgeon: preoperative, intraoperative, and postoperative issues related to intraocular pressure, cataract, andintraocular lenses. J Cataract Refract Surg. 2009 Jun;35(6):1101-20.
17. Fingeret M. Exfoliation glaucoma. Optometric Glaucoma Society Residency Education Meeting. Fort Worth, Texas. August 5, 2013.
18. Susanna R, Weinreb R Answers in Glaucoma. Rio de Janeiro, Brazil: Cultura Medica; 2005.
19. Gottanka J, Kuhlmann A, Scholz M, et al. Pathophysiologic changes in the optic nerves of eyes with primary open angle and pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2005 Nov;46(11):4170-81.
20. Gottanka J, Flügel-Koch C, Martus P, et al. Correlation of pseudoexfoliative material and optic nerve damage inpseudoexfoliation syndrome. Invest Ophthalmol Vis Sci. 1997Nov;38(12):2435-46.
21. Majka CP, Pratap C. Ophthalmic pearls: glaucoma; diagnosis and management of pseudoexfoliation glaucoma. EyeNet Magazine Online. Available at: www.aao.org/publications/eyenet/200606/pearls.cfm. Accessed June 10, 2014.
22. Dul MW. Ocular and systemic pseudoexfoliation syndrome. Optometric Management. Available at: www.optometricmanagement.com/articleviewer.aspx?articleid=71827. Accessed June 10, 2014.
23. Konstas AG, Mantziris DA, Stewart WC. Diurnal intraocular pressure in untreated exfoliation and primary open-angle glaucoma. Arch Ophthalmol. 1997 Feb;115(2):182-5.
24. Altinta O, Yüksel N, Karaba VL, Qalar Y. Diurnal intraocular pressure variation in pseudoexfoliation syndrome. Eur J Ophthalmol. 2004 Nov-Dec;14(6):495-500.
25. Bartlett J. Ophthalmic Drug Facts, 2006. Philadelphia: Wolter Kluwer Health, Inc.; 2006.
26. Desai MA, Lee RK. The medical and surgical management of pseudoexfoliation glaucoma. Int Ophthalmol Clin. 2008 Fall;48(4):95-113.
27. Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003 Jan;121(1):48-56.
28. Gracner T. Intraocular pressure response of capsular glaucoma and primary open-angle glaucoma to selective Nd:YAG laser trabeculoplasty: a prospective, comparative clinical trial. Eur J Ophthalmol. 2002 Jul-Aug;12(4):287-92.
29. Cvenkel B, Hvala A, Drnovsek-Olup B, Gale N. Acute ultrastructural changes of the trabecular meshwork after selective laser trabeculoplasty and low power argon laser trabeculoplasty. Lasers Surg Med. 2003;33(3):204-8.
30. Johnson DH. Histologic findings after argon laser trabeculoplasty in glaucomatous eyes. Exp Eye Res. 2007 Oct;85(4):557-62.
31. Realini T. Selective laser trabeculoplasty: a review. J Glaucoma. 2008 Sep;17(6):497-502.
32. Schlötzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006 May;141(5):921-37.
33. Shingleton BJ, Laul A, Nagao K, et al. Effect of phacoemulsification on intraocular pressure in eyes withpseudoexfoliation: single-surgeon series. J Cataract Refract Surg. 2008 Nov;34(11):1834-41
34. Jea SY, Francis BA, Vakili G, et al. Ab interno trabeculectomy versus trabeculectomy for open-angle glaucoma. Ophthalmology. 2012 Jan;119(1):36-42.
35. Shingleton BJ, Wooler KB, Bourne CI, O’Donoghue MW. Combined cataract and trabeculectomy surgery in eyes with pseudoexfoloation glaucoma. J Cataract Refract Surg. 2011 Nov;37(11):1961-70.
36. Landers J, Martin K, Sarkies N, et al. A twenty-year follow-up study of trabeculectomy: risk factors and outcomes. Ophthalmology. 2012 Apr;119(4):694-702.
37. Jacobi PC, Dietlein TS, Krieglstein GK. Comparative study of trabecular aspiration vs trabeculectomy in glaucomatriple procedure to treat pseudoexfoliation glaucoma. Arch Ophthalmol. 1999 Oct;117(10):1311-8.
38. Traverso CE, Bagnis A, Papadia M, Scotto R. Current and emerging medical therapies in the treatment of glaucoma. Expert Opin Emerg Drugs. 2011 Jun;16(2):293-307
39. Lankaranian D, Razeghinejad MR, Prasad A, et al. Intermediate-term results of the Ex-PRESS miniature glaucoma implant under a scleral flap in previously operated eyes. Clin Experiment Ophthalmol. 2011 Jul;39(5):421-8.
40. Jordan JF, Wecker T, van Oterendorp C, et al. Trabectome surgery for primary and secondary open angle glaucomas. Graefes Arch Clin Exp Ophthalmol. 2013 Dec;251(12):2753-60.
41. Lin SC. Endoscopic and transscleral cyclophotocoagulation for the treatment of refractory glaucoma. J Glaucoma. 2008 Apr-May;17(3):238-47.
42. Lima FE, Magacho L, Carvalho DM, et al. A prospective, comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory glaucoma. J Glaucoma. 2004 Jun;13(3):233-7.

