There are quite a few eye conditions that cause discomfort and pain, but some require immediate attention and prompt action not just from eyecare providers, but medical professionals at emergency rooms.
Retinal arterial occlusion (RAO) is one of those conditions and should be considered a true ocular emergency. This is because the retinal ischemia that is produced creates immediate and permanent cell damage—even when timely interventions are properly dispensed. The condition compromises tissues so quickly there is little likelihood of even modest functional improvements. Today, treatment extends beyond heroic ocular measures, recognizing that emergent medical interventions are required to curtail associated systemic comorbidities.
Acute retinal arterial ischemia can be caused by any process that interrupts the blood flow within the retinal arterial blood supply.1-3 Embolic events are just one mechanism; vascular compression (mass effect and acute IOP rise), arteritic and vasospastic pathologies also have the ability to interrupt arterial circulation, resulting in ischemia and permanent tissue damage.
These events can be transient or permanent and may involve the ophthalmic artery, central retinal artery or a branch retinal artery.1-3
Transient Monocular Vision Loss
Episodes of visual compromise that are reversible and last less than 24 hours are termed transient vision loss (TVL); these can be binocular or monocular.4,5 Transient monocular vision loss (TMVL) may be permanent or reversible and has an incidence of approximately 14 per 100,000 people per year.2 Patients generally report that episodes of TMVL last 20 to 30 minutes and describe it as a curtain of darkness occluding the affected eye.4,5 Specifically, TMVL is caused by occlusive arterial pathology anterior to the chiasm, at the level of the optic nerve or retina.4,5
The underlying cause of an episode is uncovered thorough history—which serves to verify timing, pattern, provoking factors and associated symptoms—laboratory testing and imaging.1-3 The most common sources of emboli consist of cholesterol, platelet-fibrin material and calcium emanating from the heart, aorta or internal carotid artery.1-3
In instances of binocular TVL, lesions are localized to the optic chiasm or retro-chiasmal visual pathway and may be the result of disease processes that involve the vasculature of both optic nerves.4,5
Sources of RAO
Non-embolic events producing TMVL are accompanied by unique patterns of signs and symptoms, including retinal migraines, which commonly last up to 20 minutes and may recur several times a day. These attacks include reversible visual phenomena such as scintillating scotoma and are frequently accompanied by headaches.4,5 Retinal vasospasm can produce a constellation of symptoms similar to those of ocular migraines without the associated headache. A relative afferent pupillary defect (RAPD) may be present during the episode, but can be resolved as perfusion is restored so long as retinal tissues are not permanently damaged.4,5
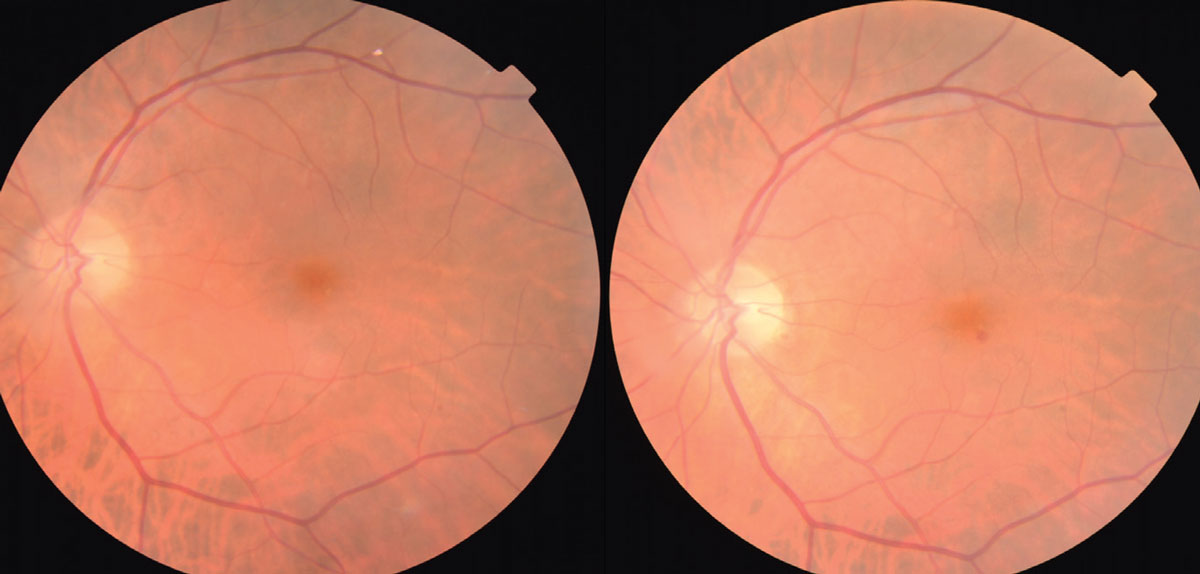 |
|
Fig. 1. The photo on the left shows a Hollenhorst cholesterol plaque within the superior-temporal vascular arcade. At follow-up three months later, the plaque is not present. Click image to enlarge. |
Giant cell arteritis (GCA) has clinical features that include headache, jaw claudication, scalp tenderness, fever, polymyalgia rheumatica and TMVL.4,5 Episodes of vision loss are of short duration—under five minutes—may be exacerbated by postural changes, can recur over a short period of time and have associated photopsia.4,5
Ocular ischemic syndrome (OIS), which results from carotid artery disease, presents with ophthalmic signs that include vascular congestion of the conjunctiva, rubeosis irides, mid-peripheral retinal hemorrhages and non-tortuous retinal veins.4,5 TMVL events associated with OIS typically have a gradual onset that lasts from seconds to minutes.4,5
Systemic hypotension and reduced cardiac output may result in hypoperfusion of the eye. In such cases, the TVL event is binocular and accompanied by lightheadedness and confusion.4,5 Other, less common diagnoses include the hypercoagulable states and orbitopathies.4,5
In contrast to TMVL, long-term or permanent vision loss associated with acute retinal arterial ischemia is the result of longer lasting partial or complete occlusion of the retinal arterial system.3,6-10 Ophthalmic artery occlusion, central retinal artery occlusion (CRAO) and branch retinal artery occlusion (BRAO) often lead to permanent visual dysfunction depending on the region of the retina involved, and may produce reduced visual acuity and noticeable visual field deficits.
The most common cause of acute retinal arterial ischemia is embolism (non-arteritic) originating from atheromatous stenosis of the ipsilateral internal carotid artery (ICA).3,6-10 Extracranial ICA stenosis of greater than 70% has been seen in up to 40% of patients diagnosed with CRAO.3,6-10 Emboli also travel from distant sources such as the heart and the aortic arch, and may consist of cholesterol, calcium or platelet fibrin aggregates.3,6-10
There are less common sources of emboli that may result in the occlusion of the retinal arterial system (Table 1). For instance, a fat embolus can occur when a fractured long bone releases droplets of fat into the bloodstream. These commonly travel to the lungs or the brain, resulting in pulmonary emboli or stroke.6-10 Septic emboli consist of bacteria or bacteria-containing tissues entering the bloodstream from a site of infection, such as an infected heart valve (infectious endocarditis).6-10 Air emboli are formed when small amounts of air enter the blood circulation during a medical procedure such as surgery or catheterization, or during injectable substance abuse.6-10
Table 1. Emboli and their common origins | |
| Type of Embolus | Origin of Embolus |
| Cholesterol | Extracranial carotid artery |
| Calcium | Valve of heart |
| Platelet fibrin aggregates | Aortic arch, artery system |
| Fat | Fat droplet from a fractured long bone |
| Septic (bacteria containing tissue) | Infected tissue (e.g., endocarditis) |
| Air | Air enters circulation via needle |
| Amniotic fluid | Enters mother’s circulation via placenta during birth |
| Foreign body | Injection of material into blood circulation, ingestion of foreign substances (e.g., cocaine, talc) |
| Paradoxical (originates from a vein) | Patent foramen ovale |
Amniotic fluid embolus—while rare—is the result of amniotic fluid entering a mother’s circulation via the placenta during childbirth.6-10 Also rare is the formation of a foreign body embolus. These emboli form as the result of a medical procedure (iatrogenic) or recreational drug abuse, such as cocaine use or additives like talc. Iatrogenic causes may also be the result of an injection during a dental procedure or from a facial cosmetic procedure where a drug or filler material is injected into a vessel.11 An embolus that originates in a vein and eventually causes the occlusion of an artery is a “paradoxical” embolus. These emboli require a pathway from the right side of the heart to the left, as in the case of a patent foramen ovale.3,6-10
Giant cell arteritis is the most common arteritic cause of retinal arterial occlusion and should be included in every vascular workup, even when suspicion is minimal.3,6-10 The doctor must be aware of the clinical features of GCA, as mentioned above, when investigating the etiology of sudden vision loss and its association with an arterial occlusive event.3,6-10 Other non-embolic causes of retinal arterial occlusion may include hematologic abnormalities resulting from sickle cell hemoglobinopathies, leukemia and systemic non-Hodgkin’s lymphoma.3, 6-10
Rapid changes in intraocular pressure have the potential to result in occlusion of the retinal arterial system by way of compression. Acutely elevated intraocular pressure in cases of glaucoma, ocular compression as a result of pressure on the eye due to positioning for an extended period of time in supine position (as in the case of spinal surgery), orbital lymphoma, retrobulbar injection and peribulbar anesthesia have all been associated with retinal arterial occlusion.1-10
Epidemiology
In the United States, CRAO has an incidence of 1.9 per 100,000 people.1-12 This incidence increases to 10.1 per 100,000 for those age 80 years and older.12 Retinal and ophthalmic artery occlusive events are marked by acute, painless monocular visual acuity and/or visual field loss, and it is only when these symptoms persist that patients make their way to either the emergency department or eye doctor.
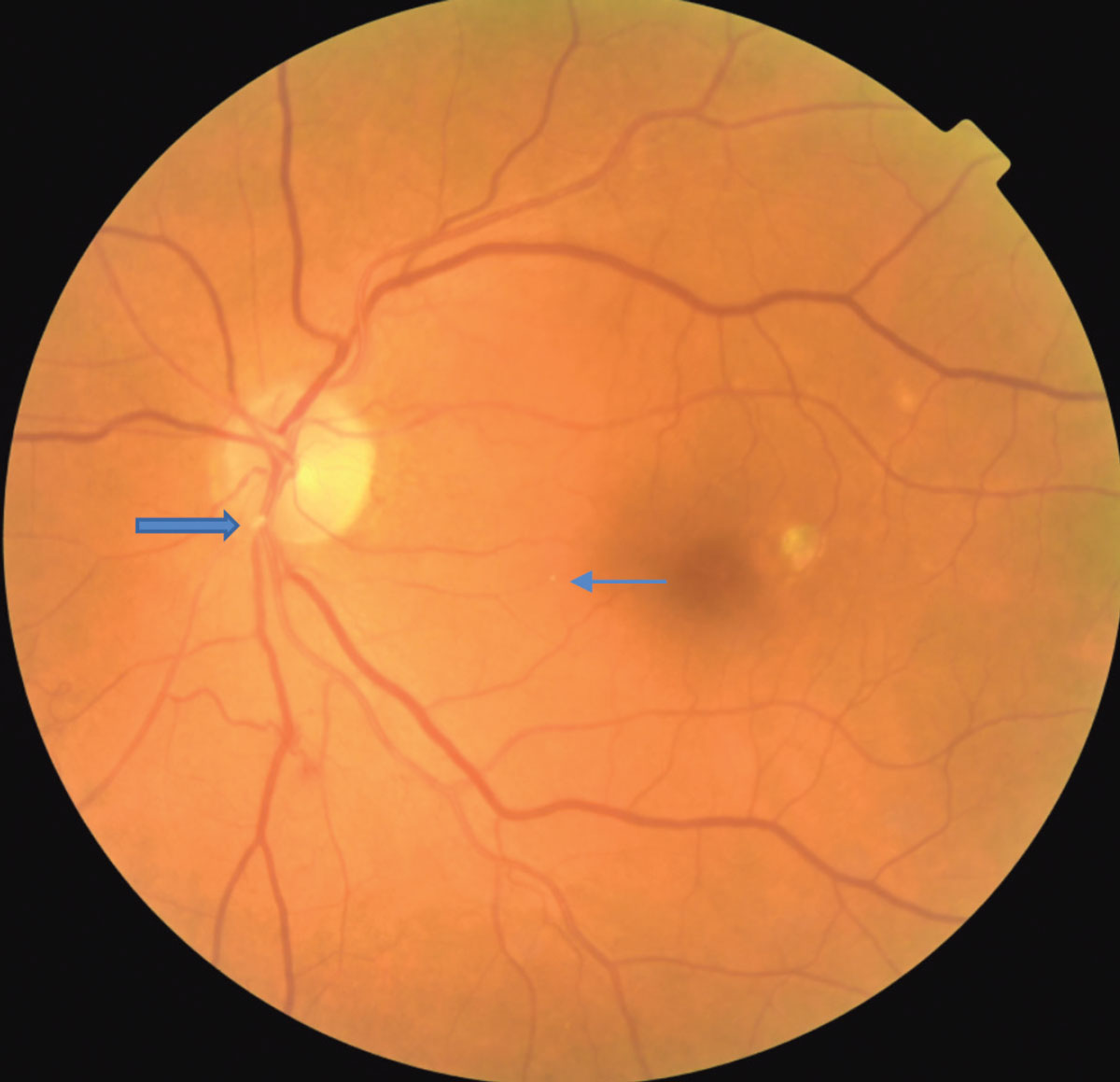 |
|
A fibrinogen platelet embolus is seen at the location of the large blue arrow. A small Hollenhorst plaque is seen at the small blue arrow. Click image to enlarge. |
Men reportedly have a higher incidence than women, and the average age of presentation is approximately 65.3,10-13 The patient’s medical history will frequently include cardiovascular disease, diabetes, hypercholesterolemia and/or a history of cigarette smoking.14,15 These systemic diseases are also confounding factors in the presence of an acute ischemic stroke.9,16,17
Signs and Symptoms
A clinical examination will confirm a precipitous reduction in visual acuity (20/400 or worse), visual field loss and an afferent pupillary defect. Color vision impairment directly correlates with the decline of visual acuity and involvement of the macular region. General medical procedures that may be performed in-office include measurements of blood pressure, pulse, pulse oximetry and assessment for carotid bruit.
Posterior segment findings are highly variable, depending on the duration of the event and the time from onset of symptoms to examination. The alteration of retinal blood vessels may include “cattle trucking” (segmentation of the blood column within the vessel) and retinal arterial attenuation.1-3,6 Other findings that may be observed include pallid edema of the optic disc, the presence of an embolus, pale and swollen retinal tissue and a cherry red-spot involving the macula.1-3,6
Pathophysiology
The posterior segment examination may reveal the presence of an embolus within an artery of the retina. In many cases, the embolus is not able to be visualized. The presentation of an embolus is helpful because it provides information regarding its consistency and origin.
A plaque that is highly reflective and white in color most likely consists of cholesterol originating from the ipsilateral carotid artery (Hollenhorst plaque). Plaques often originate from the bifurcation or within the internal carotid artery.3,6-9 Emboli consisting of calcific debris or platelet fibrin aggregates present as elongated dull grey opacities. Calcium emboli commonly originate from a heart valve. Platelet fibrin aggregates are frequently a byproduct of artery damage caused by atherosclerosis and can involve the aortic arch or the carotid or internal carotid artery.3,6-9
Emboli have the greatest likelihood of occluding the central retinal artery where its lumen is narrowest, at the point where it pierces the dura of the optic nerve, and is less likely just posterior to the lamina cribrosa.3,10 As both of these anatomical sites are behind the globe, the vast majority of emboli that cause CRAO are not able to be visualized during dilated funduscopy.3,10
Table 2. Laboratory studies and medical indications | |
| Laboratory Test | Indication for Test |
| Complete blood count (CBC) | Overall health, anemia, infection, leukemia |
| Basic metabolic panel | Measures glucose, electrolyte, kidney function |
| Prothrombin time/Partial thromboplastin time | Bleeding disorder, excessive clotting disorder |
| International normalized ration (INR) | Time to prevent formation of blood clot |
| Lipid panel | Measure of total cholesterol levels |
| Hemoglobin A1c (HA1c) | Average level of blood sugars over 60-90 days |
| Erythrocyte sedimentation rate (ESR) | Degree of inflammation present in the body |
| C-reactive protein | Measure a liver protein that responds to inflammation |
The phenomenon known as “migration of retinal emboli” can occur in both CRAO and BRAO, and may account for why emboli are not found at the time of the acute examination. In this case, by the time the patient presents to the clinic, the embolus has migrated from its original position within a retinal artery and may no longer be visible.3,10
Interestingly, calcific emboli are rough in texture and tend to get impacted within the vessel walls. Cholesterol and platelet-fibrin emboli have a less rough texture and tend to migrate easily.3,10 Therefore, in a case of monocular acute painless vision loss, a conclusion that “this is not a retinal artery occlusion” may not be made just because no retinal embolus was observed.3,10
Retinal Survival Time
Occlusion of any retinal artery ultimately results in the infarction of the retinal ganglion cells found within their vascular range.18 Research suggests that the ganglion cell layer of the retina can survive interrupted blood flow if circulation is restored in up to 97 minutes of the occlusion.18 Beyond the 97-minute mark and up to 240 minutes, more extensive and irreversible damage occurs. Occlusion beyond the 240 minutes results in catastrophic, irreversible retinal damage.18
Hayrah’s classic research of CRAO included experimental occlusion of the central retinal artery of rhesus monkeys. These experiments provided us with the 97 to 240 minute boundaries that are recited today.18 Recently, retinal survival times have been challenged based upon a review of the original research designs.10 It has been proposed that clamping the central retinal artery, as was done in the original work, would not have resulted in complete obstruction of blood to the retina. Further, it would not have eliminated the collateral circulation to the retina. In this instance, the created arterial occlusion would be incomplete. While collateral circulation would not permit complete perfusion of the retina, it certainly might have lengthened the time necessary to achieve inner retinal ischemia.10,18 This has led to thinking that retinal survival time is actually much less.
Tissue of the brain and the retina are thought to be the most energy consuming structures of the body. Comparative studies of brain and retina reveal that retinal oxygen consumption per gram is greater than that of the brain, making the retina at least as vulnerable to oxygen deprivation.19-23 Hence, opponents of the original models postulate that during complete central retinal artery occlusion, irreversible retinal ganglion cell death begins after just 12 to 15 minutes.19-23
Not all CRAOs or BRAOs are permanent and/or complete, as the involved vessels may only be partially occluded or the obstruction transient. These factors, along with the presence of a cilioretinal artery (a retinal vessel deriving its blood supply from the uninvolved choroidal circulation, present in 15% to 30% of eyes) allow for a prognosis that predicts spared sectors and potential for recovery.3,6,24 The variability in both visual acuity and spared field will correlate with the extent of the distribution of the anatomical variation of the cilioretinal artery.3,6
Ocular Management
The critical period in any case of retinal artery occlusion is from the onset of symptoms to when the patient presents for ophthalmic care. The goal of acute ocular treatment is to reverse retinal ischemia by restoring perfusion before permanent cell death occurs.1-3 Unfortunately, acute treatment remains controversial, as there is no proven standard of care.
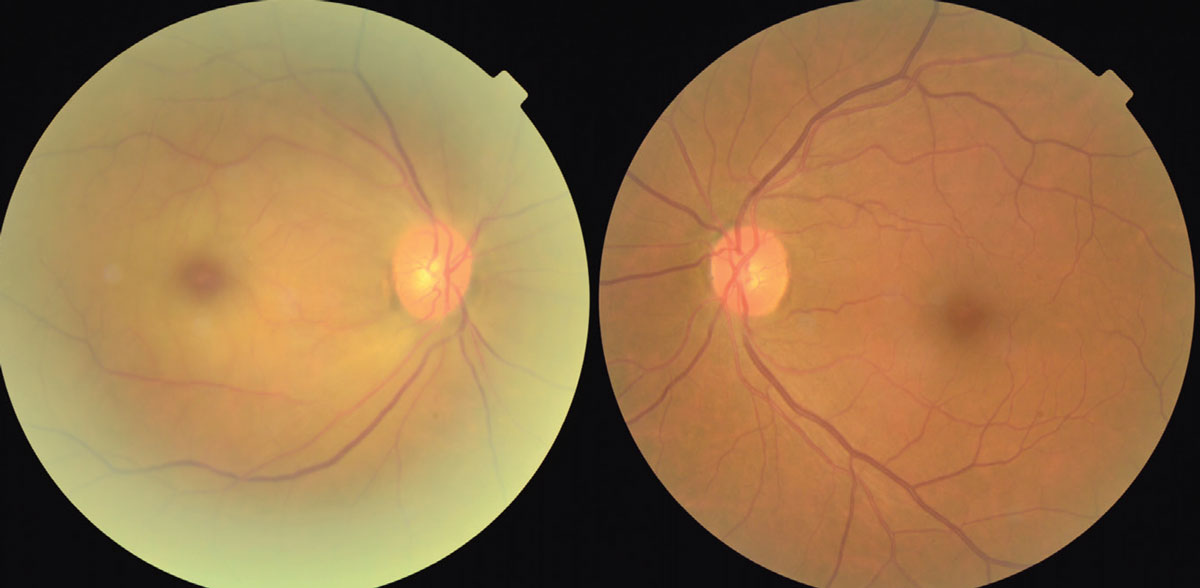 |
|
This shows the early presentation of a CRAO of the left eye with pale edema of the retina, a cherry red spot at the time of presentation of a central retinal artery occlusion. Artery attenuation is also noted. Click image to enlarge. |
The intervention of IOP lowering (topical timolol, iopidine, oral acetazolamide and IV mannitol, anterior chamber paracentesis) is directed toward decreasing the resistance to perfusion into the eye. By making it easier for blood to enter the eye, perhaps an increased flow might destabilize and move the embolus downstream or provide adequate nutrition to avert cell death.
Inhaling carbon dioxide or taking other oral or IV vasodilating agents are efforts directed toward dilation of the blood vessels of the retina. As retinal arterioles become pharmacologically enlarged, the involved arteriole might loosen its grip on an embolus, permitting it to be jettisoned downstream, restoring perfusion.
Direct pressure to the globe (“mashing” the eye or digital ocular massage) in combination with the first two interventions is intended to create a back pressure for the blood that is trying to enter the eye, such that when the direct pressure is released, an increase of blood flow might impact the embolus with such force that it dislodges the embolus, restoring retinal perfusion.3-6,8
Another treatment being explored is hyperbaric oxygen. Here, by supersaturating the red blood cells and “collateral retinal systems” (the choroidal circulation), unused oxygen might diffuse forward into the starving retina to emergently nourish it and postpone cell death.25
Unfortunately, there is little if any evidence-based data that supports any of the above therapies as a way to reverse the often catastrophic outcome.3,6,8,9
Systemic Management
A March 2021 statement from the American Heart Association formally recognized an ischemic stroke as an “episode of neurological dysfunction caused by focal cerebral, spinal or retinal infraction.”9 The common pathophysiology shared by stroke patients and retinal artery occlusion patients has prompted experts to categorize RAO as a “stroke of the eye.” This philosophy mandates concomitant and rapid triage of CRAO patients with an aggressive systemic workup, with the goal of reducing cardiac and associated central nervous system (CNS) comorbidities.9,25,26 Specifically, intravenous tissue plasminogen activator (tPA) and intra-arterial thrombolysis (IAT) are commonly used in the management of occlusive ischemic cerebral infarcts and are being more closely investigated as a more standardized treatment of CRAO.9,26,27
Intravenous fibrinolysis most commonly uses the infusion of alteplase (which acts as a tPA) and is proven to be most efficacious when administered within 4.5 hours of the onset of symptoms.26,27 A meta-analysis of observational studies found that patients with acute CRAO treated with this modality had a 50% rate of clinical recovery (visual acuity of 20/100 when initial acuity was 20/200) when treated within 4.5 hours of onset of symptoms.28 Unfortunately, treatment must be used selectively, as symptomatic intracranial hemorrhage can occur. To reduce this risk, confounding factors such as active bleeding, recent stroke or hemorrhage or use of anticoagulation therapy should be identified.9,28
IAT involves the introduction of the thrombolytic agent (tPA) directly into the ophthalmic circulation via microcatheterization.29 The advantage is that therapy is delivered to the thrombus, limiting systemic circulation and reducing the risk of intracranial and systemic hemorrhage.29 Inherent risks include possible arterial dissection, catheter-induced spasm and dislodgement with distal embolization of an atheromatous plaque withing the ophthalmic circulation.29
Prompt triage and referral to an emergency department, with the goal of admission to a stroke floor for a comprehensive medical assessment by experts who understand this syndrome, is now the standard of care.1,2,9,27 Laboratory studies should include a complete blood count, basic metabolic panel, prothrombin time/partial thromboplastin time, international normalized ratio, lipid panel, hemoglobin A1c, erythrocyte sedimentation rate and C-reactive protein (Table 2). These studies assess the overall wellness of the patients and target the most common vascular risk factors (HTN, DM, hyperlipidemia) that may lead to other vascular occlusive events or myocardial infarction.1-3
Diagnostic imaging should be ordered in all cases of retinal vascular occlusion to search for the potential origin of an embolus. If vascular narrowing or blockage exists, it must be identified to eliminate the potential for subsequent occlusive events in the brain or heart.7-9,14 Imaging studies include computed tomography (CT), magnetic resonance imaging (MRI), CT angiogram, MR angiogram, carotid doppler, transthoracic echocardiogram and transesophageal echocardiogram. Ambulatory cardiac rhythm monitoring is frequently performed to diagnose atrial fibrillation.7-9,14
Treatment goals on the stroke floor include tight management of vascular risk factors that have a common impact on CRAO, stroke and carotid artery disease.7,14 Control of hypertension (the leading risk factor for retinal ischemia), hyperlipidemia (the second most common risk factor associated), diabetes and obstructive sleep apnea are of most importance. Lifestyle changes and education include smoking cessation, exercise and dietary restrictions to reduce obesity and BMI.7-9,14
Prognosis
Prompt recognition of the signs and symptoms associated with acute retinal ischemia secondary to retinal artery occlusion play a critical role in the preservation of visual function and prevention of stroke and cardiovascular events. It is has been reported that 25% of patients presenting with CRAO have had a “silent” stroke that is visible upon MRI, with no patient awareness or symptoms.1,2 The risk of an ischemic neurologic event occurring within months following a CRAO is 2.7 times higher when compared to control subjects.9
Studies have documented a range of 7.4% to 24.2% of patients having a stroke in the first four years following a CRAO.30-33 The incidence of acuter coronary syndrome in patients with CRAO 70 years of age and higher was 2.48 times higher versus aged matched controls.34 The lifetime of CRAO patients who do not make lifestyle changes has been estimated to be reduced by 10 years vs. healthy controls.6-9
Summary
Historically, even the most heroic ophthalmic treatments rarely result in the restoration of functional vision. Acknowledging the association between cardiac and central nervous system conditions makes retinal ischemic events a true medical emergency and the need to refer cases of retinal ischemia to the emergency department must not be understated. Eyecare providers should have a plan that incorporates the primary care provider, the emergency department and a stroke center into the global care of the patient with CRAO.
Dr. Myers is senior staff optometrist at Coatesville Veterans Affairs Medical Center in Coatesville, PA. He is also a guest lecturer and adjunct clinical faculty at the Pennsylvania College of Optometry. Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is also attending medical staff in the department of ophthalmology at Albert Einstein Medical Center in Philadelphia. They have no financial interests to disclose.
1. Biousse V, Nahb F, Newman NJ. Management of acute retinal ischemia. Ophthalmol. 2018;125(10):1597-1607. 2. Dattilo M, Newman NJ, Biousse V. Acute retinal arterial ischemia. Ann Eye Sci. 2018;3(6):1-19. 3. Hayreh SS. Central retinal artery occlusion. Indian J Ophthalmol. 2018;66(12):1684-94. 4. Feroze KB, O’Rourke MC. Transient vision loss. StatPearls, NCBI bookshelf. www.ncbi.nim.gov/books/NBK430845. 5. Fitzpatrick T, Gocan S, Wang CQ, et al. How do neurologist diagnose transient ischemic attack: A systematic review. Int J Stroke. 2019;14(2):115-24. 6. Chen CS, Varma D, Lee A. Arterial occlusions to the eye: from retinal emboli to ocular ischemic syndrome. Asia Pac J Ophthalmol. 2020;9(4):349-57. 7. Schorr EM, Rossi KC, Stein LK, et al. Characteristics and outcomes of retinal artery occlusions: Nationally representative data. Stroke. 2020;51(3):800-07. 8. Lee KE, Tschoe C, Coffman SA, et al. Management of acute central retinal artery occlusion, a “Retinal Stroke”: An institutional series and literature review. J Stroke Cerebrovasc Dis 2021;30(2):1-9. 9. Mac Grory B, Schrag M, Biousse V, et al. Management of central retinal artery occlusion: A scientific statement from the American Heart Association. Stroke. 2021 March 8. Epub ahead of print. 10. Tobalem S, Schutz JS, Chronopoulos A. Central retinal artery occlusion – rethinking retinal survival time. BMC Ophthalmol 2018;18(1):1-6. 11. Lee JS, Kim JY, Jung C, et al. Iatrogenic ophthalmic artery occlusion and retinal artery occlusion. Prog Retin Eye Res. 2020 March 10, 2020. Epub ahead of print. 12. Leavitt JA, Larson TA, Hodge DO, et al. The incidence of central retinal artery occlusion in Olmstead County, Minnesota. Am J Ophthalmol. 2011;152(5):820-3. 13. Lawlor M, Perry R, Hunt BJ, et al. Strokes and vision: The management of ischemic arterial disease effecting the retina and the optic lobe. Surv Ophthalmol 2015;60(4):296-309 14. Callizo J, Feltgen N, Pantenburg S, et al. Cardiovascular risk factors in central retinal artery occlusion: Results of a prospective and standardized medical examination. Ophthalmol 2015;122(9):1881-88. 15. Long CP, Chan AX, Bakhoum CY, et al. Prevalence of subclinical retinal ischemia in patients with cardiovascular disease – a hypothesis driven study. EClinicalMedicine. 2021. 16. Fallico M, Lotery AJ, Longo A, et al. Risk of acute stroke in patients with retinal artery occlusion: a systematic review and meta-analysis. Eye 2020; 34(4):683-89. 17. Park SJ, Choi NK, Yang BR, et al. Risk and risk periods for stroke and acute myocardial infarction in patients with central retinal artery occlusion. Ophthalmol 2015; 122(11): 2236-43. 18. Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina 2007;27(3):276-89. 19. Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia – the ischemic penumbra. Stroke. 1981;12(6):723-5. 20. Raichle ME. The pathophysiology of brain ischemia. Ann Neurol 1983;13(1):2-10. 21. Lee VM, Grab MC, Zipfel GJ, et al. Brain tissue response to ischemia. J Clin Invest 2000;106(6):723-31. 22. Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121(4):547-57. 23. Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularized and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20(2):175-208. 24. Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res 2005;24(4):493-519. 25. Masters TC, Westgard BC, Hendriksen SM, et al. Case series of hyperbaric oxygen therapy for central retinal artery occlusion. Retinal cases and brief reports. 2019;00(00):1-6. 26. Feltgen N, Neubauer, Jurklies B, et al. Multicenter study of the European Assessment Group for Lysis in the Eye (EAGLE) for the treatment of central retina l artery occlusion: design issues and implications. Eagle Study report no. 1. Graefe’s Arch Clin Exp Ophthalmol. 2006;244(8):950-56. 27. Mac Grory B, Nackenoff A, Spitzer MS, et al. Intravenous fibrinolysis for central retinal artery occlusion: A cohort study and updated patient-level meta-analysis. Stroke. 2020;51(7):2018-25. 28. Schrag M, Youn T, Schindler J, et al. Intravenous fibrinolytic therapy in central retinal artery occlusion: a patient-level meta-analysis. JAMA Neurol. 2015;72(10):1148-54. 29. Hakim N, Hakim J. Intra-arterial thrombolysis for central retinal artery occlusion. Clin Ophthalmol. 2019;13:2489-2509. 30. Hankey GI, Slattery JM, Warlow CP. Prognosis and prognostic factors of retinal infarction: a prospective cohort study. BMJ. 1991;302(6775):499-504. 31. Chang YS, Jan RL, Weng SF, et al. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: a nationwide population-based study. Am J Ophthalmol. 2012;154(4):645-52. 32. Avery MB, Magal I, Kherani A, et al. Risk of stroke in patients with ocular arterial occlusive disorders: a retrospective Canadian study. J Am Heart Assoc. 2019;8(3):e010509. 33. Lee J, Kim SW, Lee SC, et al. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion-weighted magnetic resonance imaging study. Am J Ophthalmol. 2014:157(6):1231-8. 34. Chang YS, Chu CC, Weng SF, et al. The risk of acute coronary syndrome after retinal artery occlusion: a population-based cohort study. Br J Ophthalmol 2015;99(2):227-31. |

