 |
A 44-year-old male presented with a chief complaint of moderate blurred vision in his left eye over several months and requested an updated prescription. He reported that he has needed glasses from a young age. His last eye exam was six months prior. His medical history was unremarkable.
On examination, his best-corrected visual acuity was 20/20 OD and 20/60 OS. The right eye measured -8.50 +0.50 x 175 and the left eye was -9.25 DS.
Extraocular motility testing was normal. Confrontation visual fields were full to careful finger counting OU. Pupils were equally round and reactive, without a relative afferent pupillary defect. The anterior segment was unremarkable. His intraocular pressure measured 17mm Hg OU.
Dilated fundus exam revealed obliquely inserted optic nerves with peripapillary atrophy in both eyes. Images of the right and left eye are available for review (Figures 1 and 2). Additionally, SD-OCT and fluorescein angiography was obtained (Figure 3). A late frame from the FA is available for review (Figure 4).
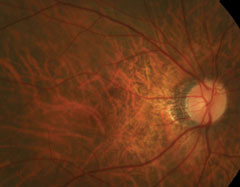 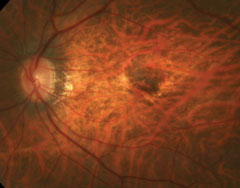 |
| Figs. 1 and 2. Right and left eye of our patient—note the changes in the posterior pole of the left eye. |
Take the Quiz
1. How would you characterize the SD-OCT findings documented in his left eye?
a. Cystoid macular edema.
b. Vitelliform lesion.
c. Choroidal neovascularization type 2.
d. Central serous retinopathy.
2. What additional testing would be helpful in establishing a diagnosis?
a. Visual field.
b. Visual evoked potential.
c. Standardized ultrasound.
d. Multifocal electroretinography.
3. What is the correct diagnosis for this patient?
a. Wet macular degeneration.
b. Macular telangiectasia.
c. Choroidal rupture.
d. Myopic choroidal neovascularization.
4. How should this patient likely be treated?
a. Laser photocoagulation.
b. Photodynamic therapy.
c. Anti-VEGF injection.
d. Combination photodynamic therapy and anti-VEGF therapy.
For answers, see below.
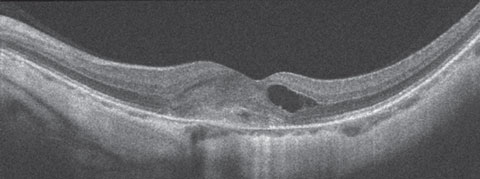 |
| Fig. 3. SD-OCT of the left eye. Can you determine the cause of this presentation? |
Diagnosis
Our patient has developed choroidal neovascularization (CNV) as a result of his high myopia. The diagnosis of pathological myopia does not have a universal definition, but is most commonly described as an eye with an axial length greater than 26.5mm and/or at least -6D of refractive error accompanied by degenerative changes of the sclera, choroid and retinal pigment epithelium (RPE) specific to axial elongation.1-3 These degenerative changes can include a tessellated fundus, posterior staphyloma, RPE atrophy, Fuchs’ spots (macular degenerations due to myopia), lacquer cracks (breaks in Bruch’s membrane), peripheral retinal thinning (lattice) and detachment, foveoschisis and CNV.1,3,4 Pathologic myopia is also associated with increased incidence of cataract and optic nerve anomalies.4
Pathologic myopic CNV (mCNV) affects 5.2% to 11.3% of pathological myopic patients and is the leading cause of CNV in patients younger than 50 years of age.1,3,5 mCNV is one of the most visually threatening complications of pathologic myopia, as it diminishes central vision.1
When examining a patient with high myopia, look for predisposing factors for the development of mCNV. These include patchy chorioretinal atrophy over diffuse atrophy, lacquer cracks and posterior staphyloma. On clinical examination, mCNV presents as a small (< 1 disc area), flat, grayish subretinal membrane. A hyperpigmented border is a sign of chronicity.1,3 Additionally, up to 30% of people with mCNV in one eye will develop mCNV in the other eye, and thus close monitoring of the non-involved eye is indicated.1
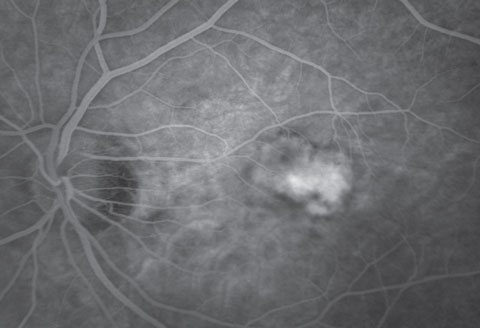 |
| Fig. 4. Late frame of the fluorescein angiogram. |
Optical coherence tomography (OCT) is often enough to confirm and monitor mCNV. The CNV net in mCNV is typically type 2, meaning it is subretinal and located largely above the RPE. Because it is above the RPE a grayish membrane can often be visualized on clinical exam. This is in contrast to age-related macular degeneration, where the CNV net is most commonly sub-RPE (type 1 CNV) and the features of the CNV are not visible.
In our patient, we are able to see the dark circular features of the CNV. This is confirmed on the SD-OCT where a higher reflective lesion is seen above the RPE. Anterior to that, cystoid macular edema has developed.
Fluorescein angiography (FA) for mCNV displays early hyperfluorescence with minimal late leakage, as was the case with our patient.
Treatment
Our patient received an anti-vascular endothelial growth factor (anti-VEGF) intravitreal injection of Avastin (bevacizumab, Genentech). There are several theories as to why anti-VEGF therapy is effective. The most commonly reported is that a break in Bruch’s membrane (sometimes called lacquer cracks) induces cellular changes in the RPE that release VEGF.2 Additionally, in vitro studies have shown that the mechanical stretch of RPE cells up-regulates pro-angiogenic factors, including VEGF.2,4 The REPAIR study, the largest multicenter study for mCNV treated with Lucentis (ranibizumab, Genentech), found that the average improvement of BCVA at 12 months was 13.8 letters.6 Similar findings were seen with Eylea (aflibercept, Regeneron) and Avastin.7,8
Our patient’s vision improved and stabilized at 20/30 after three intravitreal Avastin injections. Unfortunately, four years later fluid started to accumulate and vision declined to 20/70. Despite repeated anti-VEGF injections, the vision continued to decline to 4/200 in the left eye. Thankfully, the right has had no significant pathologic myopia complications and remains 20/20.
This case was written by Leslie Small, OD, optometric resident at Bascom Palmer Eye Institute.Retina Quiz Answers:
1) c; 2) c; 3) d; 4) c.
|
1. Chan N, Teo K, Cheung C. Epidemiology and diagnosis of myopic choroidal neovascularization in Asia. Eye Contact Lens 2016;42:48-55. 2. Zhang Y, Han Q, Ru Y, et al. Anti-VEGF treatment for myopic choroid neovascularization: from molecular characterization to update on clinical application. Drug Des Devel Ther. 2015;9:3413-21. 3. Silva R. Myopic maculopathy: a review. Ophthalmologica. 2012;228:197-213. 4. Cho B, Shin J, Yu H. Complications of Pathologic Myopia. Eye Contact Lens. 2016;42:9-15. 5. Munk M, Ruckert R, Zinkernagel M, et al. The role of anti-VEGF agents in myopic choroidal neovascularization: Current standards and future outlook. Expert Opin Biol Ther. 2015. 6. Tufail A, Narendran N, Patel P, et al. Ranibizumab in myopic choroidal neovascularization: the 12-month results from the REPAIR study. Ophthalmology. 2013;120:1944-5.e1. 7. Pece A, Milani P, Monteleone C, et al. A randomized trial of intravitreal bevacizumab vs. ranibizumab for myopic CNV. Graefes Arch Clin Exp Ophthalmol. 2015;253:1867-72. 8. Ikuno Y, Ohno-Matsui K, Wong T, et al. Intravitreal Aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR study. Ophthalmology 2015;122:1220-7. |

