
|
Early in our training and careers, we often avoided diagnosing patients with optic neuritis. When the prototypical patient came to us (e.g., a young person, often female, complaining of color vision loss, blurred vision and significant pain upon eye movement, and manifesting a relative afferent pupillary defect but a normal optic disc appearance), we were hesitant to make a confirmatory diagnosis of optic neuritis––though it often was our suspicion.
Our reluctance stemmed from the strong association of optic neuritis with multiple sclerosis (MS). While we were able to use magnetic resonance imaging (MRI) to identify characteristic MS brain lesions, we rarely did so. The reason was that, even if we did confirm that optic neuritis was the first manifestation of MS, there was nothing we could do to treat the patient.
Usually, optic neuritis would spontaneously improve and had a good visual prognosis. However, there was no accepted treatment for MS at that time. We knew that if we definitively diagnosed optic neuritis, the underlying implication of MS could pose a hardship for patients who––at that time––could have lost or not qualified for medical or disability insurance because carriers might suspect they could be on the hook for a disabling disease. Thus, we took a “don’t ask, don’t tell” policy.
Today, fortunately, the climate is much different. Over the past several years, research has made great advances in immunomodulatory therapy that can diminish the disabling effects of MS. Not only are these therapies beneficial in reducing disability, but the early initiation of therapy offers the best patient outcomes.
|
|
|
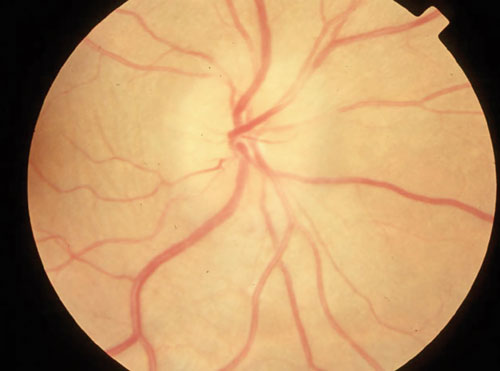
| Swollen optic disc in a patient with optic neuritis and MS. |
What is Optic Neuritis and MS?
Optic neuritis is an acute, inflammatory, demyelinating event of the optic nerve that may be idiopathic and localized to the optic nerve, or may be or become associated with other systemic illnesses––notably MS. Optic neuritis often is the initial presenting sign of MS.1-3
After five years, clinically definite multiple sclerosis (CDMS) develops in 30% of patients who present with optic neuritis, although the degree of neurological impairment is generally mild at that point.4
The typical patient with demyelinating optic neuritis is a young female, often between the ages of 20 and 40. In 92% of cases, the vision loss is painful––particularly upon eye movement.5
Multiple sclerosis is an acquired, multifactorial, autoimmune inflammatory demyelinating disease that affects the white matter located in the central nervous system (CNS). MS is defined by recurrent bouts of CNS inflammation that damage both the myelin sheath surrounding the axons and the axons themselves. There is a predilection for specific areas of the CNS, including the optic nerve and periventricular white matter of the cerebellum, brain stem, basal ganglia and spinal cord. Myelin is responsible for insulating the nerves of the peripheral and central nervous system, permitting speeding of electrical impulses along nervous tissues.
Evidence suggests that the cellular immune response contributes to the degradation of myelin. This patchy demyelination likely is caused by a deposition of mononuclear cells, such as macrophages and B-cells in perivascular regions.6 Loss of myelin greatly slows nervous conduction and leads to the neurological deficits seen in MS.
On average, patients experience clinical relapses every one to two years during the so-called relapsing/remitting phase of the disease––although serial MRI suggests that inflammatory lesions are practically continuous throughout this period.
Current MS Therapies
MS frequently is treated using immunomodulatory agents. The major drugs used in the United States include interferon beta-1b (Betaseron, Bayer HealthCare) and interferon beta-1a (Avonex, Biogen Idec; and Rebif, Pfizer). Glatiramer acetate (Copaxone, Teva) also is frequently used.7
• Betaseron is injected subcutaneously. The recommended dose is 0.25mg every other day. It is supplied as 0.3mg of lyophilized powder in a single-use vial for reconstitution.
• Avonex is FDA approved to treat relapsing forms of MS, decrease the number of disease recurrences and to slow the onset of associated physical disabilities. It is indicated for use in people who have experienced a first attack and have lesions consistent with MS via MRI.
Avonex is a once-weekly treatment that is available in three dosage forms: a prefilled syringe; a single-use, prefilled autoinjector that uses a covered needle that’s 50% shorter than the standard Avonex needle; and an unmixed form designed to be used with a standard Avonex syringe.
• Rebif is administered three times per week, and also is available in three dosing forms: Rebif Rebidose, a preassembled, prefilled, single-use autoinjector; the Rebiject II autoinjector, which works with the Rebif prefilled syringe and is designed to automate the injection process; and a ready-to-use, preassembled, prefilled syringe.
• Copaxone is a synthetic analogue of the MS-associated antigen, myelin basic protein. Copaxone 40mg is an injectable therapy that is taken three times per week. Copaxone is available as both a prefilled syringe and an autoinjector.
Is Early Treatment Better?
Numerous studies have evaluated the effectiveness of immunomodulatory therapies in reducing disease recurrence, accumulation of disability and progression to CDMS. Additionally, multiple research groups have sought to determine if the timing of therapeutic initiation has a clinical effect on the severity of MS development and progression.
Betaseron’s effects on MS are currently unknown, but are thought to block T cells from attacking myelin. However, the Betaseron in Newly Emerging Multiple Sclerosis for Initial Treatment (BENEFIT) study showed that early treatment with beta-1b significantly delayed the time to a second flare-up and conversion to CDMS compared with placebo.8
The Controlled High Risk Avonex Multiple Sclerosis Study (CHAMPS) looked at whether the initiation of Avonex in patients experiencing a first-time demyelinating event, such as optic neuritis, and who also had MRI brain abnormalities suggestive of prior demyelinating events could delay or prevent progression to CDMS.9
Its three-year results were very impressive.9 Development of CDMS occurred in 35% of patients using Avonex and in 50% of placebo-treated patients. Additionally, patients who used Avonex experienced a decrease in the number and size of new MRI brain lesions compared to those in the placebo group.9
A 10-year extension of the CHAMPS study looked at whether immediate initiation of treatment at the time of a clinical demyelinating event in patients at high risk for CDMS (those with an abnormal brain MRI) could alter long-term disease progression.10
The immediate-treatment group exhibited a 40% lower rate of CDMS, as well as a reduced relapse rate. Also, few patients in the immediate treatment group reached the Expanded Disability Status Scale milestone scores of 4.0 or greater (9% of patients) or 6.0 or greater (6% of patients). The researchers believed that immediate initiation of Avonex at the time of a clinically isolated syndrome (such as optic neuritis) in high-risk patients reduced relapse rates by more than 10 years, and was associated with better clinical outcomes.10
In the PreCISe study, patients who used Copaxone experienced a significantly reduced rate of, and longer conversion time to, CDMS than those who took a placebo––with a 45% incidence reduction overall, and a 66% reduction if optic neuritis was the presenting finding.11
More recently, the same research team noted that initial treatment with Copaxone reduced CDMS conversion risk by 41% compared to delayed-treatment, and was associated with a 972-day delay in conversion to CDMS. They concluded that the reduced rate of CDMS conversion, as well as lower MRI measures of disease activity and lesion burden, support initiating Copaxone treatment soon after the first clinical symptoms suggestive of MS manifest.12 Further, they recommend continuing treatment to sustain benefits.12
Clearly, there has been a paradigm shift in the way that we diagnose and manage patients with optic neuritis and other conditions associated with MS development. While eye care practitioners are not going to manage patients with MS, we frequently are the individuals to diagnose conditions associated with the disease.
Before, we never rushed to make the diagnosis of optic neuritis and MS because there were no interventions available to help patients. Now, the immunomodulatory therapies available have clearly shown to reduce the burden of MS, and that early treatment can delay the onset and taper the severity of CDMS.
When encountering optic neuritis, internuclear ophthalmoplegia or any condition associated with MS, the treatment paradigm dictates prompt diagnosis with a risk assessment and referral to a physician skilled in the management of patients with MS. This will ensure that appropriate intervention will be started to provide patients with the maximum benefit.
1. Chan JW. Optic neuritis in multiple sclerosis. Ocul Immunol Inflamm. 2002;10(3):161-86.
2. Soderstrom M. Optic neuritis and multiple sclerosis. Acta Ophthalmol Scand. 2001 Mar;79(3):223-7.
3. Pirko I, Blauwet LK, Lesnick TG, et al. The natural history of recurrent optic neuritis. Arch Neurol. 2004 Sep;61(9):1401-5.
4. Optic Neuritis Study Group. The five-year risk of MS after optic neuritis. Experience of the optic neuritis treatment trial. Neurology. 1997 May;49(5):1404-13.
5. Beck RW. Optic neuritis study group. Neurologic impairment 10 years after optic neuritis. Arch Neurol. 2004 Sep;61(9):1386-9.
6. Confavreux C, Vukusic S. Accumulation of irreversible disability in multiple sclerosis: from epidemiology to treatment. Clin Neurol Neurosurg. 2006 Mar;108(3):327-32.
7. Plosker GL. Interferon1b: a review of its use in multiple sclerosis. CNS Drugs. 2011 Jan;25(1):67-88.
8. Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006 Oct 10;67(7):1242-9.
9. Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med. 2000 Sep 28;343(13):898-904.
10. Kinkel RP, Dontchev M, Kollman C, et al. Association between immediate initiation of intramuscular interferon beta-1a at the time of a clinically isolated syndrome and long-term outcomes: a 10-year follow-up of the Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance. Arch Neurol. 2012 Feb;69(2):183-90.
11. Comi G, Martinelli V, Rodegher M, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet. 2009 Oct 31;374(9700):1503-11.
12. Comi G, Martinelli V, Rodegher M, et al. Effects of early treatment with glatiramer acetate in patients with clinically isolated syndrome. Mult Scler. 2013 Jul;19(8):1074-83.

