 |
A 20-year-old Hispanic female presented as an emergency office visit with complaints of progressive drooping in her left upper eyelid of 11 months’ duration. She noted it seemed to worsen by the end of the day, particularly after computer use. She denied ocular pain, headache, diplopia, muscle weakness, difficulty swallowing and breathing. Her medical, ocular, social and family histories were unremarkable. Unaided visual acuity was 20/20 OD and OS. Interpalpebral apertures were 7mm OD and 5mm OS. Marginal reflex distance was 5mm OD and 3mm OS, and levator function was 15mm OD and 12mm OS. Pupils were equal, round and reactive to light without relative afferent defect; they measured 6mm and 3mm in dim and bright illumination in both eyes. She demonstrated 10/10 color plates in each eye. Intraocular pressure measured 13mm Hg OD and 16mm Hg OS. Confrontation testing was normal. With the exception of left upper eyelid ptosis and mild, incomitant left abduction deficit, the anterior segment exam was unremarkable.
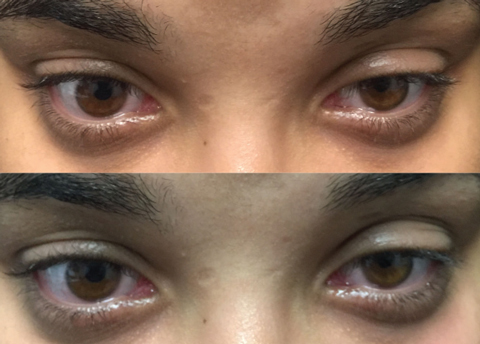 |
| Top photo: Left lid before ice test. Bottom: Same lid after test, showing minimal improvement. |
Oh, MG!
Patients presenting with variable weakness of the eyelids and eye muscles who show no signs of cranial nerve palsy raise flags for myasthenia gravis (MG). The patient also fit the demographic profile for the condition, occurring most commonly in women under age 40. As she demonstrated key symptoms in her left eyelid, we considered ocular myasthenia gravis (OMG) and ran a series of in-office tests to narrow and confirm a diagnosis. Aim to employ these when working up an OMG suspect:
• Lid-fatigability test. This quick, non-invasive exam helps determine whether and to what degree the affected lid experiences fatigue. Ask the patient to sustain a prolonged upgaze fixation and observe eyelid position. Our patient’s left ptotic lid showed considerable increase in ptosis after one minute. The orbicularis muscle was also assessed.
• Cogan’s lid twitch. Next, we asked her to look down and then move the direction of gaze back up to her primary position.1 If a patient overshoots upward before returning to their resting position, they’ve tested positive—as ours did.
• Eyelid retraction. Another telltale sign of OMG manifests in eyelid retraction, occurring when one lid rests in ptotic position and the other retracts. As Hering’s law says, yoked muscles work synergistically. So, while the ptotic eyelid sits low, the patient is constantly using the orbicularis muscle to raise the affected lid. This extended effort causes over-action of the contralateral, unaffected eyelid, which will manifest as contralateral lid retraction. Manually closing the ptotic eyelid resolves the contralateral lid retraction. Our patient tested positive.
• Orbicularis “squeeze test.” Ask the patient to forcefully close their eyes while you attempt to open them. In our patient, both eyelids were easily opened despite her strong attempt to squeeze them shut. This confirmed bilateral weakness of the orbicularis muscle. Version testing further revealed subjective horizontal diplopia during extreme left gaze. A basic cover test carried out in the nine cardinal positions of gaze found her orthophoric in all positions, except for six prism diopters of left esotropia in extreme left gaze.
• The sleep and ice tests. At home, the patient can self-administer the sleep test by closing the eyes for 30 minutes and then noting whether there is any improvement in ptosis, diplopia or both. If so, there is a strong indication for OMG. The ice test involves placing an icepack over the affected eyelid for two to five minutes.2 If there is at least a 2mm elevation, or improvement, of the ptosis, the test is positive. Cooling the eye reduces acetylcholinesterase (AchE), thereby increasing acetylcholine (Ach) within the neuromuscular junction (NMJ). The sensitivity and specificity of the ice test is 76.9% and 98.3%, respectively.3
Running both tests produces a larger change in lid position than the sleep test alone.4 Here, we ordered the ice test to evaluate improvement in the left upper ptosis and diplopia, which confirmed ocular symptoms of OMG.
• Pharmacologic testing. Edrophonium (Tensilon) prevents the breakdown of Ach by inhibiting AChE within the NMJ. In the so-called Tensilon test, following administration of the drug, OMG patients will typically demonstrate improved muscle strength in either the levator or the affected extraocular muscle (EOM). The sensitivity of the Tensilon test is 95% in generalized myasthenia gravis (GMG) and 86% for OMG.5 Tensilon must be administered by a provider licensed to perform intravenous injections, typically a neurologist. If the diagnosis is still in question following the Tensilon test, neostigmine—which has a longer duration of action—can be given as an intramuscular injection by appropriately qualified staff.2
• Neurostimulation tests. Repetitive nerve stimulation studies and single-fiber electromyography (SFEMG) involve stimulation of nerve bundles and recording action potentials. SFEMG is the more sensitive test for detecting abnormal neuromuscular transmission. It has an 85% to 100% sensitivity for OMG when used on the frontalis or orbicularis muscle, and a sensitivity of 91% to 100% in GMG.7,8
Communication BreakdownMyasthenia gravis is an autoimmune disease of the NMJ, the site of communication between nerve bundles and muscle fibers. It causes classical symptoms of variable muscle weakness worsened by fatigue. MG may affect any age group but is less likely in patients over age 70 and more common in females.2,10 There are two forms of the disease: generalized and ocular. The former involves the bulbar, limb and respiratory muscles.2 The latter is a subtype confined to the EOMs, levator muscle and orbicularis oculi.2 In all forms of MG, antiacetylcholine receptor antibodies (AChR-Abs) block receptors on muscle fibers from receiving Ach molecules. This results in defective signal transmission and poor muscle contraction. Because EOMs have fast-twitch fibers that require constant binding of Ach to muscle fibers, the earliest stages of the disease may present with variable ptosis and diplopia. These are the initial signs in more than 50% of MG patients.11 Within two years, 50% to 80% of those affected with OMG will convert to GMG.12,13 In addition to ptosis and orbicularis weakness, ocular motility deficits are common symptoms of OMG. All EOMs may be affected, with the medial rectus and superior rectus showing up as the most common.14 The motility pattern is often variable and fatigable but can mimic nerve palsies, gaze palsies or internuclear ophthalmoplegias.11 Additional testing of saccades may reveal intrasaccadic fatigue and a decline in saccadic velocity.15 |
Next Steps
Given our patient’s progressive, fatigable left upper lid ptosis, incomitant left abduction deficit, orbicularis muscle weakness, positive ice test, normal pupils and lack of respiratory or peripheral muscle weakness, we diagnosed OMG. We then ordered AChR-Ab levels as well as thyroid function tests, since thyroid disease can accompany MG, and referred the case to neurology. Two weeks later, the neurologic exam confirmed our findings by Tensilon testing.
Treatment aims to improve both general and ocular muscle weakness, achieve disease remission and slow or prevent progression to GMG.9 Corticosteroids are the most commonly used medication for both OMG and GMG. Our patient is being managed on a regimen of 100mg pyridostigmine (an AChE inhibitor) and 20mg oral prednisone.
Despite treatment, many patients still exhibit bothersome diplopia, ptosis or both. Supportive measures include temporary prism or occlusion therapy. Surgery can be considered in patients whose symptoms are stable for a minimum of six months and immunosuppressive therapy can be considered in all patients with MG as monotherapy or adjunct therapy with corticosteroids and AChE inhibitors.
The optometrist’s role is to monitor disease status and manage the ophthalmic symptoms with prism, occlusion therapy or surgical referral.
1. Keane JR. Vertical diplopia. Semin Neurol. 1986;6:147–54. |

