Omega fatty acid supplements are some of the most widely used nonvitamin, nonmineral products on the market today. According to one study, 7.8% of adults and 1.1% of children ages four to 17—nearly 20 million people in all—have used a fish oil supplement in the previous 30 days.1,2 Researchers defined the supplement as either a fish oil, omega-3, docosahexaenoic acid (DHA) or eicosapentaenoic acid (EPA) fatty acid.
Research shows fish oil supplementation can reduce blood pressure and inflammation, increase brain blood flow and provide structural strength for neurons.3-7 As the compounds are a natural and expected component of our bodies and diets, they have a wide margin of safety.8 Although humans evolved consuming a diet with roughly equal amounts omega-3 and omega-6 essential fatty acids, in today’s western diet the ratio of omega-6 to omega-3 fatty acids ranges from approximately 20-30:1, and the amounts of saturated and trans fatty acids, all of which are inflammatory, have increased sharply.8
Because omega-3 fatty acids decrease inflammation and promote health, they can provide significant benefits for patients with dry eye—a disease inflammatory in nature.9 A closer look at omega fatty acids will help you better understand their use in dry eye and give you the tools necessary to educate your patients on their benefits.
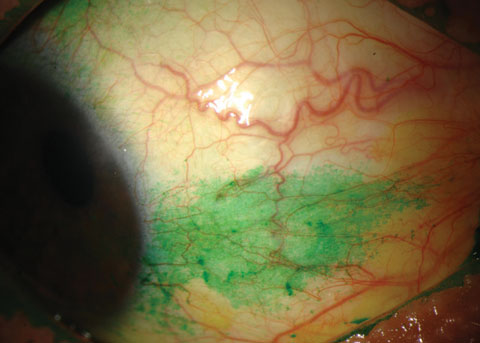 |
| Conjunctival staining with lissamine green in a dry eye patient. Photo: Michelle Hessen, OD. |
Inflammation Modulation
Omega-3 and omega-6 fatty acids, both dietary polyunsaturated fatty acids (PUFAs), are essential nutrients—they cannot be synthesized in the body and must be obtained from the diet. Omega-3s are incorporated into cell membranes in all tissues of the body.13 Diet-induced changes in the polyunsaturated fatty acid composition of a cell membrane have an impact on the cell’s function, partly because these fatty acids represent a reservoir of molecules that perform important signaling or communication roles within and between cells.13 Further, omega-3s compete with omega-6s for incorporation into all cell membranes, which depends on dietary intake.10,11
Arguably, arachidonic acid (ARA), an omega-6, is most important. When cells are activated by external stimuli, ARA is released from cell membranes and transformed into proinflammatory cellular mediators such as thromboxanes, prostaglandins and leukotrienes.12 Arachidonic acid metabolism is the target of nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and celecoxib, and leukotriene antagonists (e.g., montelukast). Dietary omega-3s displace ARA from membranes and compete with it for the enzymes that catalyze the biosynthesis of thromboxanes, prostaglandins and leukotrienes.9 This is considered to be one of the main mechanisms of their anti-inflammatory effects.13 Thus, omega-3s limit cells such as monocytes, neutrophils and eosinophils from synthesizing the arachidonic acid-derived mediators of inflammation.13
In addition, enriching membranes with omega-3s can modulate cellular signaling events, membrane protein function and gene expression.13 Research shows alpha-linolenic acid (ALA) dramatically reduces prostaglandin formation, probably by downregulating the transcription of genes coding for proinflammatory mediators such as c-reactive protein and IL-6.9,14 EPA exerts much of its anti-inflammatory benefit by suppressing NF-KappaB activation, a protein complex that controls transcription and cytokine production, which reduces the elaboration of proinflammatory mediators.15
DHA and EPA are the precursors to docosatrienes and resolvins, which downregulate proinflammatory IL-1 gene expression, inhibit TNFα and reduce neutrophil entry to sites of inflammation.9,16 EPA and DHA can be synthesized from ALA (and are more potent), but this process does not reliably or efficiently occur in humans.13,17 Dietary oils rich in ALA, although having beneficial effects of their own, usually do not reproduce the biological activity associated with dietary fish oils.13
GLA and DGLA
Not all omega-6s are proinflammatory. Gamma-linolenic acid (GLA) is known as the most powerful health-promoting omega-6, and is found in evening primrose oil, borage seed oil, hemp oil and black currant seed oil.9 GLA is elongated to form the biologically activated dihomo-γ-linolenic acid (DGLA), which goes on to either form the anti-inflammatory prostaglandin E1 or it forms anti-inflammatory ARA.9
Research shows GLA can be beneficial in dry eye therapy, but it should not be given without EPA/DHA. Only one enzymatic step is necessary to convert the anti-inflammatory DGLA to proinflammatory ARA, and EPA competes against DGLA to ARA conversion, ensuring DGLA forms prostaglandin E1.9, 27
Fatty Acids & Dry Eye
Since supplementing with healthy fatty acids causes competition and displacement of proinflammatory fatty acids from the cell membranes, a strong case has been made for proper fatty acid supplementation in inflammatory diseases such as dry eye. Fatty acids affect gene expression by multiple complex mechanisms.9 In an animal model, resolvins and protectins reverse corneal epithelial damage associated with dry eye, increase tear flow, promote healthy epithelia and decrease cyclooxygenase-2 (COX-2) expression and the infiltration of macrophages.18
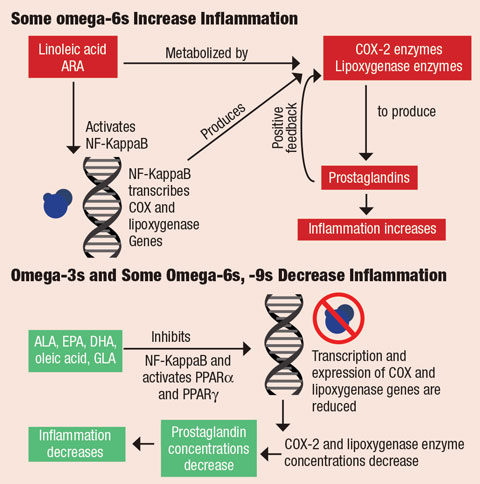 |
| In omega-6s, transcription factor NF-KappaB (blue) is activated by linoleic acid and ARA. NF-KappaB binds to the genes’ promoter regions that code for cyclooxygenase and lipoxygenase, responsible for forming prostaglandins. Formation of prostaglandins activates the inflammatory cascade. In omega-3s and some omega-6s and -9s, fatty acids ALA, EPA, DHA, oleic acid and GLA NF-KappaB activity and activate peroxisome proliferator-activated receptors (PPARα and PPARg) in the nucleus of cells. This decreases concentrations of COX-2 and lipoxygenase proteins, which decreases the concentration of prostaglandins and, ultimately, decreases inflammation. Click image to enlarge. |
Research also shows omega-3s inhibit the inflammatory properties established in moderate to severe dry eye disease such as: increased T-cell infiltration, increased tear inflammatory cytokines, increased ocular surface human leukocyte antigen (HLA)-DR and increased intercellular adhesion molecule expression.19-21
Investigators find the anti-inflammatory effect of omega-3s on dry eye disease is similar to that of cyclosporine. In a 2011 study, dry eye participants were given a combination of omega-3s and the omega-6 GLA, which was shown to improve tear film break-up time (TBUT) and relieve patient symptoms; however, the addition of topical cyclosporine did not convey any statistically significant improvement in TBUT beyond that achieved by the supplement alone.22
In a recent study, researchers studied the relationship between lipid profiles in human tears and dry eye symptoms and signs, and found that both omega-3 and omega-6 lipid pathways are activated in the human tear film.23 Results show the ratio of omega-6 to omega-3 tear lipids is elevated in dry eye patients in proportion to the degree of tear film dysfunction and corneal staining.23 They also found a significant correlation between the tear film omega-6 to omega-3 ratio and tear volume (Schirmer score), as well as tear stability and corneal staining.23 These findings are consistent with prior studies that show how changes in tear volume correlate with dietary omega-3 intake, and improvements of dry eye signs occur with omega-3 supplementation.24-26
Prescribing Essentials
While data show the benefits of fatty acids—for instance, meta-analyses show omega-3s improve TBUT and Schirmer scores—no consensus exists on the dose, composition, length of treatment or form used of omega-3s or omega-6s for dry eye therapy.23,27-29 Consider the following when deciding on a treatment:
Not all studies are the same. Clinicians should take into consideration the results and methodologies of well-designed studies. For instance, many studies use olive oil—oleic acid—as a placebo to study the effects of omega-3 therapy. Olive oil is not a biologically inert substance, as it provides anti-inflammatory benefits itself.30 While some study results look promising, the methodology may influence expectations.
Triglyceride vs. ethyl ester omega-3s. Omega-3s occur naturally in the triglyceride (Tg) form. For purification and concentration, manufacturers create fatty acid ethyl esters (EEs) by replacing the glycerol backbone of a Tg and substituting it with ethanol. The resulting EEs allow manufacturers to perform fractional distillation to concentrate the long-chain fatty acids at lower temperatures. Most commercially available over-the-counter fatty acid supplements, as well as the prescription-only Lovaza (omega-3-acid ethyl esters, GlaxoSmithKline), are in the EE form. Research shows pancreatic lipase enzymes break down EE to a lesser extent than Tg, explaining the poorer absorption of EE forms.31 Studies also show that if the EE form is taken with food, particularly a meal high in fat, its absorption increases significantly.32 Finally, studies show differences in the stability of EE vs. Tg forms, with EE breaking down and oxidizing more quickly than the Tg form.33,34
Some manufacturers convert the EE form back to the natural Tg form using food-grade enzymes. This process, called glycerolysis, removes the ethanol molecule and re-esterifies the EPA and DHA fatty acids to a glycerol backbone, forming a re-esterified Tg. This step adds to the cost, but it creates a better tolerated and more easily absorbed product.31
Dosage. The ideal omega-6 to omega-3 ratio in the diet should be less than 4:1.8 Since western diets have a much higher ratio, higher doses of omega-3 supplementation are usually safe. No consensus in the literature exists regarding the optimal dose for dry eye therapy. For adults with coronary disease, the American Heart Association (AHA) recommends a daily omega-3 fatty acid supplement of 1g EPA and DHA.35 For adults with high cholesterol, the AHA recommends a daily supplementation of 2g to 4g of EPA and DHA.35
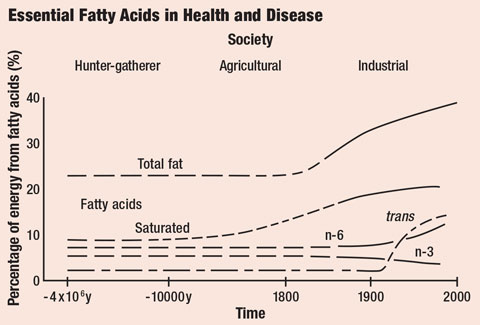 |
| Saturated and trans fat intake has increased substantially in recent history. Click image to enlarge. |
In a recent study, 105 participants were given either 2,240mg of re-esterified omega-3s daily or a placebo of safflower oil. The treatment group showed statistically significant improvement in tear osmolarity, omega-3 index levels, TBUT, Matrix metallopeptidase 9 (MMP-9) and ocular surface disease index (OSDI) symptom scores.36 Based on the wide margin of safety of the fatty acids and dosages used in the literature, a reasonable starting dose is between 1,000mg and 2,250mg of a combination of EPA and DHA daily.36-39
Contraindications and precautions. The biological effects of omega-3 and omega-6 fatty acids may pose some risks to patients:
- GI tract. High doses of fish oil may cause loose stool, diarrhea, “fishy burps” and other gastrointestinal side effects, but these are more common with the more difficult to digest EE form.
- Bleeding. EPA and DHA therapeutic doses usually start at 1,000mg per day, but more than 3g per day may increase the risk of bleeding.35 Therefore, take into context the patient’s overall procoagulant vs. anticoagulant balance before prescribing. Ask patients if they are on blood thinners or taking supplements known to have an anticoagulant effect such as garlic, ginkgo or saw palmetto.
- Immunosuppression. While suppressing inflammatory responses with omega-3s benefits those with inflammatory or autoimmune diseases, it may decrease the immune system’s ability to destroy pathogens.40 One study comparing immune cell function ex vivo at baseline and after supplementing with omega-3s (mainly EPA and DHA) demonstrated immunosuppressive effects.40 While it’s unclear if these findings translate to impaired immune function in vivo, exercise caution should in individuals with compromised immune systems.
Label. The two most important numbers on a fatty acid supplement label are the actual omega-3 content, expressed in milligrams of EPA and DHA, and the number of pills in a serving size. Also, fatty acids are no different than any other poorly regulated dietary supplement category and are prone to inaccurate label claims and contamination. Several independent labs conduct periodic reviews of commercially available supplements to audit their omega-3 content and assess their purity.
Combination therapy. Since fatty acids compete for space in cell membranes, supplementation with a single fatty acid can exacerbate depletion of other fatty acids. For instance, supplementation with EPA and DHA reduces DGLA.41 Therefore, GLA should also be supplemented in addition to EPA and DHA. An adequate dose of GLA would be 500mg per day. 9
Research continues to highlight health benefits of fatty acid therapy’s. While more research is needed to set specific dosing guidelines in dry eye, supplementing with reliable brands offers patients relief from dry eyes and other health benefits beyond the ocular surface.
Dr. Poteet is a certified nutrition specialist, fellow and vice president of the Ocular Nutrition Society. She has a special interest in autism research.
|
1. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015 Feb 10;(79):1-16. 2. Black LI, Clarke TC, Barnes PM, et al. Use of complementary health approaches among children aged 4-17 years in the United States: National Health Interview Survey, 2007-2012. Natl Health Stat Report. 2015 Feb 10;(78):1-19. 3. Fredman G, Serhan CN. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem J. 2011;437(2):185–97. 4. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–61. 5. Jackson PA, Reay JL, Scholey AB, Kennedy DO. DHA-rich oil modulates the cerebral haemodynamic response to cognitive tasks in healthy young adults: a near IR spectroscopy pilot study. Br J Nutr. 2012;107(8):1093–8. 6. Litman BJ, Niu SL, Polozova A, Mitchell DC. The role of docosahexaenoic acid containing phospholipids in modulating G protein-coupled signaling pathways: visual transduction. J Mol Neurosci. 2001;16(2–3):237–42. 7. Mitchell DC, Niu SL, Litman BJ. Enhancement of G protein-coupled signaling by DHA phospholipids. Lipids. 2003;38(4):437–43. 8. Simopoulos, AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70(suppl):560S-9S. 9. Vasquez A. Reducing pain and inflammation naturally. Part II: new insights into fatty acid supplementation and its effect on eicosanoid production and genetic expression. Nutritional Perspectives: J Counc Nutr Am Chiro Assoc. 2005;28(1):5-16. 10. Calder PC. n-3 polyunsaturated fatty acids, inflammation and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S-19S. 11. Healy DA, Wallace FA, Miles EA, et al. Effect of low to moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids. 2000;35(7):763-8. 12. Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871-5. 13. Surette ME. The science behind dietary omega 3 fatty acids. Can Med Assoc J. 2008;178(2):177-80. 14. Adam O, Wolfram G, Zollner N. Effect of alpha-linolenic acid in the human diet on linoleum acid metabolism and prostaglandin biosynthesis. J Lipid Res. 1986;27(4):421-6. 15. Zhao Y, Joshi-Barve S, Barve S, et al. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23(1):71-8. 16. Hong S, Gronert K, Devchand PR, et al. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acids in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278(17):14677-87. 17. Francois CA, Connor SL, Bolewicz LC, et al. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr. 2003 Jan;77(1):226-33. 18. Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther. 2010;26(5):431-9. 19. Epstein SP, Gadaria-Rathod N, Wei Y, et al. HLA-DR expression as a biomarker of inflammation for multicenter clinical trials of ocular surface disease. Exp Eye Res. 2013;111:95-104. 20. Wei Y, Gadaria-Rathod N, Epstein S, et al. Tear cytokine profile as a noninvasive biomarker of inflammation for ocular surface diseases: standard operating procedures. Invest Ophthalmol Vis Sci. 2013;54(13):8327-36. 21. Massingale ML, Li X, Vallabhajosyula M, et al. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28(9):1023-7. 22. Jackson MA, Burrell K, Gaddie I, Richardson SD. Efficacy of a new prescription omega only medical food supplement in alleviating signs and symptoms of dry eye, with or without concomitant cyclosporine A. Clin Ophthalmol. 2011;5:1201-6. 23. Walter SD, Gronert K, McClellan AL, et al. Omega 3 tear film lipids correlate with clinical measures of dry eye. Invest Ophthalmol Vis Sci. 2016;57(6):2472-8. 24. Harauma A, Saigon J, Watanabe Y, et al. Potential for daily supplementation of n-3 fatty acids to reverse symptoms of dry eye in mice. Prostaglandins Leuko Essential Fatty Acids. 2014;90(6):207-13. 25. Kawakita T, Kawabata F, Tsui T, et al. Effects of dietary supplementation with fish oil on dry eye syndrome subjects: randomized controlled trial. Biomed Res. 2013;34(5):215-20. 26. Kangari H, Eftekhari MH, Sardari S, et al. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology. 2013;120(11):2191-6. 27. Hom MM, Asbell P, Barry B. Omegas and dry eye: more knowledge, more questions. Optom Vis Sci. 2015;92(9):948-56. 28. Liu A, Ji J. Omega-3 essential fatty acid therapy for dry eye syndrome: a meta-analysis of randomized controlled studies. Med Sci Monit. 2014;20(1):1583-9. 29. Zhu W, Wu Y, Li G, et al. Efficacy of polyunsaturated fatty acids for dry eye syndrome: a meta-analysis of randomized controlled trials. Nutr Rev. 2014;72(10):662-71. 30. Massaro M, Carluccio MA, De Caterina R. Direct vascular antiatherogenic effects of oleic acid: a clue to the cardioprotective effects of the Mediterranean diet. Cardiologia. 1999;44(6):507-13. 31. Dyerberg J, Madsen P, Moller JM, et al. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot Essent Fatty Acids. 2010;83(3):137-41. 32. Visioli F, Rise P, Barassi MC, et al. Dietary intake of fish vs formulations leads to a higher plasma concentrations of n-3 fatty acids. Lipids. 2003;38(4):415-8. 33. Yoshi H, Furuta T, Siga H, et al. Autoxidation kinetic analysis of docosahexaenoic acid ethyl ester and docosahexaenoic triglyceride with oxygen sensor. Biosci Biotechnol Biochem. 2002;66(4):749-53. 34. Song JH, Inoue Y, Miyazawa T. Oxidative stability of docosahexaenoic acid-containing oils in the form of phospholipids, triacylglycerols, and ethyl esters. Biosci Biotechnol Biochem. 1997;61(12):2085-8. 35. Krauss RM, Eckel RH, Howard B, et al. AHA Scientific Statement: AHA dietary guidelines revision 2000: a statement for healthcare professionals from the nutrition committee of the American Heart Association. Circulation. 2000;102(18):2284-99. 36. Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35(9):1185-91. 37. Olenik A. Effectiveness and tolerability of dietary supplementation with a combination of omega-3 polyunsaturated fatty acids and antioxidants in the treatment of dry eye symptoms: results of a prospective study. Clin Ophthalmol. 2014;8:169-76. 38. Bhargava R, Kumar P, Kumar M, et al. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. Int J Ophthalmol. 2013;6(6):811-6. 39. Olenik A, Jimenez-Alfaro I, Alejandro-Alba N, et al. A randomized, double-masked study to evaluate the effect of omega-3rd fatty acids supplementation in meibomian gland dysfunction. Clin Interv Aging. 2013;8:1133-8. 40. Fenton JI, Hord NG, Ghosh S. Immunomodulation by dietary long chain omega-3 fatty acids and the potential for adverse health outcomes. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2013;89(6):379-90. 41. Cleland LG, Gibson RA, Neumann M, et al. The effect of dietary fish oil supplement upon the content of dihomo-gammalinolenic acid in human plasma phospholipids. Prostaglandins Leukot Essent Fatty Acids. 1990;40(1):9-12. |

