AMD is already the leading cause of blindness and vision loss in the elderly. An estimated 7.2 million Americans have some form of AMD, yet most of them won’t know about it until they begin to suffer vision loss.1 Making this news all the more somber, treatments available for our patients today—especially those intended for wet AMD—are much better than they were 10 or 20 years ago. If only these individuals could be brought into the fold sooner, they’d maintain their central vision longer, as well as the quality of life that comes with it.
 And while future treatments—whether in topical, oral or nanoparticle form—will be more convenient and likely better than even today’s best options, we still need to be on the front lines of prevention, diagnosis and early intervention.
And while future treatments—whether in topical, oral or nanoparticle form—will be more convenient and likely better than even today’s best options, we still need to be on the front lines of prevention, diagnosis and early intervention.
We generally think of AMD patients as those with drusen, retinal pigment epithelial changes and/or choroidal neovascular membranes (CNVMs). Although these are indeed the hallmark clinical signs we use to diagnose AMD, could we be detecting it sooner? Could we be more efficiently assigning risk to younger patients to help prevent disease from ever starting? For patients who have AMD, could we predict which are more likely to ultimately have their vision compromised by the disease?
While the clinical exam is our “gold standard,” this article discusses different techniques that may help us to quantify the risk of AMD development, detect the disease sooner in its course—even before clinical visibility—and determine the risk of eventual vision loss among those who already have AMD.
History and Clinical Exam
Family history and some simple lifestyle-related questions are helpful in assigning risk. For instance, a patient with a first-degree relative with AMD is at greater risk than someone without a positive family history.
Other risk factors are high glycemic index diet, being overweight, smoking, poor diet and other lifestyle options that are similar to risk factors for cardiovascular disease.2 While it seems fairly obvious, remember that age is another important risk factor: The older we get, the more likely we are to develop AMD, as its name implies.
In the clinical exam, we may be able to take a closer look at the macula if we use either newer lenses that allow for higher digital resolution, or simply higher-magnification lenses than what we may have used in the past. For example, a 60D lens provides approximately 20% more magnification than a 78D lens, and a newer lens, such as Digital Widefield by Volk, can provide 20% more field of view than a traditional 78D or 90D lens.
Macular Pigment Optical Density
When seeing patients in the office, measuring macular pigment optical density (MPOD) may be one way to help gauge risk of developing AMD. Macular pigment helps protect the photoreceptors from oxidative stress caused by such offenders as ultraviolet and blue light.3 In general, the more dense the MPOD, the “healthier” the macula.
MPOD is easily measured in the office with one of several commercially-available instruments. The two most commonly used clinical devices are the Quantifeye (Zeavision) and the Macuscope (Marco Ophthalmic). Although a relatively small percentage of eye care providers use such technology, the numbers are growing, especially as some practices choose to create “AMD centers of excellence” within their offices. One potential advantage of MPOD is that it can be measured over time, which can be used to gauge response to treatment. Research has shown that improvement of MPOD is likely to improve visual function, as documented by a number of different metrics.4
A low MPOD score is considered to be below 0.21, moderate falls between 0.21 and 0.44, and high is 0.45 or above. For patients identified with low MPOD, there are a variety of options to increase pigmentary volume. The most likely approach is dietary modification and/or vitamin supplementation.
 Increased intake of the carotenoids lutein and zeaxanthin is essential in improving MPOD scores. To have a significant clinical impact, though, adequate amounts are key. Many believe that daily intake of at least 6mg of combined lutein and zeaxanthin should be considered the minimum. Patients not on a carotenoid-based, eye-specific supplement are unlikely to achieve optimal levels of these compounds—unless they eat plenty of spinach, kale and/or broccoli every day.
Increased intake of the carotenoids lutein and zeaxanthin is essential in improving MPOD scores. To have a significant clinical impact, though, adequate amounts are key. Many believe that daily intake of at least 6mg of combined lutein and zeaxanthin should be considered the minimum. Patients not on a carotenoid-based, eye-specific supplement are unlikely to achieve optimal levels of these compounds—unless they eat plenty of spinach, kale and/or broccoli every day.
Dark Adaptation
Once a patient has developed AMD, we may be able to detect it before anatomical changes become clinically evident. One test with high sensitivity and specificity for macular degeneration is dark adaptation. This functional test measures how long it takes the retina to adjust from bright to dim light. Dark adaptation has been shown to decrease in patients with early to late AMD; it correlates well with visual symptoms related to night vision.5,6
A new instrument, the AdaptDx (Maculogix), has been FDA approved as a dark adaptometer for vision research and drug development. Although not yet expressly approved to diagnose AMD, AdaptDx is more accurate as a diagnostic evaluation for AMD than the other tests such as contrast sensitivity and visual acuity we use in clinical care.7
This instrument can both be used to identify the presence of AMD through a screening test and also to monitor disease progression or stabilization with a staging test. The concept is similar to glaucoma detection by performing a screening visual field, then doing a threshold field and repeating it over time to monitor treatment efficacy.
Perhaps in the not-too-distant future, dark adaptation may be used to gauge the clinical efficacy of anything from nutritional intervention in early AMD to stabilization in wet AMD following anti-VEGF injection; studies currently underway are using it as an endpoint.
OCT
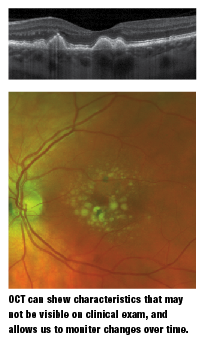
Optical coherence tomography (OCT) has become part of the standard of care in retina patients, especially in macula-related diseases. OCT can show subtle changes that are not appreciated clinically and, more importantly, it can allow us to monitor changes over time. Anything from areas of RPE disruption by drusen to the thickness of individual drusen can be precisely measured and tracked.
Because OCT is an objective and quantifiable test, analysis of change over time is fairly simple. Also important is the ability to detect small pockets of fluid that may not be easily observed on clinical evaluation yet are indicative of wet AMD.
We know from studies that the sooner that CNVM is found and the smaller it is when treatment begins, the more likely a patient is to have a better visual outcome with treatment, as found in the MARINA study.8
FAF
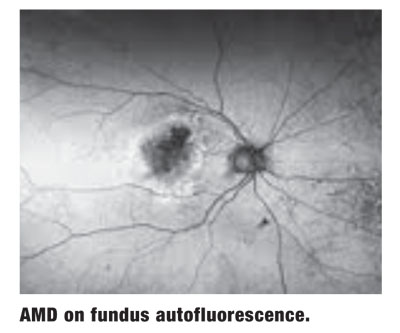
Another imaging technology that has definite applications in early AMD detection is fundus autofluorescence (FAF). This technology evaluates the metabolic activity of the RPE, helping the clinician to determine whether it is considered normal, overactive or underactive.
Specifically, when there is hyper-autofluorescence, it is a sign of metabolically overactive RPE that is “sick,” dysfunctional or potentially dying. By contrast, hypo-autofluorescence is a sign of underactive, or likely dead, RPE. This is important from a number of perspectives, not the least of which is measuring areas of geographic atrophy from one visit to the next in an effort to monitor for change and/or treatment efficacy.
An area of hyper-autofluorescence can also show where a lesion is “active” and where vision is likely to be affected next if successful treatment is not employed.9
Interestingly, FAF gives us a different “picture” of AMD than other tests; it is functional rather than structural imaging. There have been several studies showing correlation between peripheral lesions and central AMD.10 This prompts the question: Is AMD inherently a disease affecting the posterior pole, or is it rather a condition that manifests itself in a visually significant way in the macula/posterior pole? As it turns out, the more peripheral FAF, the more likely advanced AMD is to occur.10
Furthermore, unique patterns of peripheral FAF may predict which final type and stage of AMD a patient is likely to develop.10 For example, mottled FAF is correlated to RPE depigmentation in the macula. Knowing what to expect may help you establish appropriate follow-up schedules and/or improve patient education.
Genetic Testing
Once we have identified that a patient has AMD, we need to concentrate not only on treatment-related decisions but also on appropriate education and follow-up. For the majority of patients—specifically, those with nonexudative AMD—counseling about lifestyle choices (e.g., nutrition and smoking) will likely take center stage.
 During the patient education process, it is important to discuss the benefits of careful monitoring at home as well as in the office. However, how can we know which patients are at greater risk, and therefore need more careful follow-up or more targeted home monitoring beyond an Amsler grid?
During the patient education process, it is important to discuss the benefits of careful monitoring at home as well as in the office. However, how can we know which patients are at greater risk, and therefore need more careful follow-up or more targeted home monitoring beyond an Amsler grid?
Genetic testing now offers a bit of a window into an AMD patient’s potential future. In the past, all we could do was to ask about a family history of AMD, but without really knowing its specific genetic correlation to our patient. That has changed with the advent of commercially-available genetic testing. For example, we can now test specific single nucleotide polymorphisms (SNPs) that a patient possesses in order to gauge risk of change to advanced AMD.
Genetic tests for AMD primarily evaluate SNPs of complement factor H (CFH) and age-related maculopathy susceptibility (ARMS) 2. However, a number of other SNPs are looked at to gauge future risk on a two-, five- and 10-year timeframe.11 Indeed, when known markers are used, then the risk of advanced AMD can be predicted with a probability (C statistic) score of 0.83.12
Generally speaking, if a patient has a diagnosis of drusen, dry AMD or wet AMD, their insurance should cover the cost of genetic testing to help assess the likelihood of progression to advanced AMD.
One reason that genetic testing is potentially valuable and relatable to patients is that it is essentially predictive of vision loss—their top priority. We also know that, beyond genetics, modifiable risk factors can affect outcomes.13
Another potential important role for genetics is the area of pharmacogenetics, or the emerging science of choosing treatments based on genetic profiles. Several papers either directly or indirectly address whether nutritional therapy may have an effect on patients with specific genetic findings.14 The basic idea is that certain individuals benefit tremendously from something that actually may be harmful to others, based on their genetics. Although some of the literature on this is controversial, there is little argument that genetics plays an important role in AMD development and response to therapy.14 For instance, recent research shows that certain genotypes are more likely to respond favorably to Lucentis (ranibizumab, Genentech) than other genetic subtypes.15 Similar research is being conducted using Avastin (bevacizumab, Genentech) and Eylea (aflibercept, Regeneron).
The use of genetic subtypes to guide treatment decisions is an example of personalized medicine. The idea that treatments could be designed to match specific risks and benefit profiles based on individual characteristics (including genetics) will likely lead to better outcomes. This is especially important for those at highest risk for advanced AMD with a poor outcome.
Home Monitoring
Once an individual is deemed to be at high risk, either due to genotype or phenotype, it is likely that the patient should be followed more closely. This may entail more frequent office visits and/or more attentive home monitoring by the patient (and doctor). Historically, we have provided our patients with a home Amsler grid test. Even though Amsler grid is neither very sensitive nor specific, and subject to numerous user error variables, it has been the best at-home option.
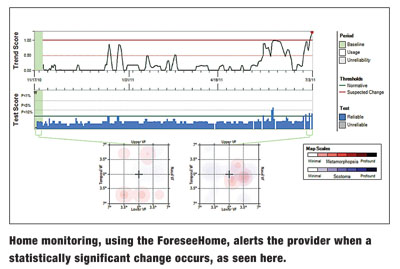
Potentially beneficial in some circumstances is a more sensitive and specific option for patients.16 The ForeseeHome device (Notal Vision) detects and monitors central and paracentral metamorphopsia indicative of choroidal neovascularization in AMD. Using the device at home, patients can test their vision electronically as frequently as they want, which is remotely overseen by a monitoring center. The monitoring center is like an extension of the doctor’s office—if there is any deviation from baseline/normal, the center immediately notifies the prescribing doctor.
This test has been granted some validation through its use in the AREDS2 study, which showed that more patients are diagnosed with their CNVM at 20/40 or better and patients who developed CNVM and using ForeseeHome had better results than those that were not using it.17 We have long known that a better presenting acuity when CNVM is diagnosed correlates with better acuity following treatment, and we specifically know this to be true with anti-VEGF injections.18
Regardless of which tests are used for AMD, there are now more options to evaluate macular structure and function. With more metrics to assess, we will have the ability to make an earlier and more accurate diagnosis of AMD, as well as determine the patient’s likely visual prognosis. With this information, we can better arm our patients with individualized knowledge and treatments so that they can potentially take a more active and beneficial role in their care.
Dr. Gerson practices at Grin Eyecare in Olathe, Kan. He is on the advisory boards for ArcticDx and Maculogix.
1. Klein R, Chou CF, Klein BE, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011 Jan;129(1):75-80.
2. Erke MG1, Bertelsen G, Peto T, et al. Cardiovascular risk factors associated with age-related macular degeneration: the Tromsø Study. Acta Ophthalmol. 2014 Jan 25.
3. Thompson LR, et al. Elevated retinal zeaxanthin and prevention of light induced photoreceptor cell death in quail. Invest Ophthalmol and Vis Sci. 2002;43:3538-3549.
4. Richer SP, Stiles W, Graham-Hoffman K, et al. Randomized, double-blind, placebo controlled study of zeaxanthin with atrophic age-related macular degeneration: The Zeaxanthin and Visual Funtion Study (ZVF). Optometry. 2011 Nov;82(11):667-680.
5. Jackson GR, Scott IU, Kim IK, et al. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014 Mar 10;55(3):1427-31.
6. Scilley K, Jackson GR, Cideciyan AV, et al. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002 Jul;109(7):1235-42.
7. Owsley C, Jackson GR, White M, et al. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001 Jul;108(7):1196-202.
8. Boyer DS, Antoszyk AN, Awh CC, et al; MARINA Study Group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007 Feb;114(2):246-52.
9. Querques G, Querques L, Forte R, et al. Precursors of type 3 neovascularization: a multimodal imaging analysis. Retina. 2013 Jun;33(6):1241-8.
10. Tan CS, Heussen F, Sadda SR. Peripheral autofluorescence and clinical findings in neovascular and non-neovascular age-related macular degeneration. Ophthalmology. 2013 Jun;120(6):1271-7.
11. Perlee LT, Bansal AT, Gehrs K, et al. Inclusion of genotype with fundus phenotype improves accuracy of predicting choroidal neovascularization and geographic atrophy. Ophthalmology. 2013 Sep;120(9):1880-92.
12. Seddon J, Reynolds R, Maller J, et al. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009 May;50(5):2044-53
13. Sobrin L, Seddon JM. Nature and nurture—genes and environment—predict onset and progression of macular degeneration. Prog Retin Eye Res. 2014 May;40C:1-15.
14. Awh CC, Lane AM, Hawken S, et al. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2013 Nov;120(11):2317-23.
15. Hermann MM, Van Asten F, Muether PS, et al. Polymorphisms in vascular endothelial growth factor receptor 2 are associated with better response rates to ranibizumab treatment in age-related macular degeneration. Ophthalmology. 2014 Apr;121(4):905-10.
16. Isaac DL, Avila MP, Cialdini AP. Comparison of the original Amsler grid with the preferential hyperacuity perimeter for detecting choroidal neovascularization in age-related macular degeneration. Arq Bras Oftalmol. 2007 Sep-Oct;70(5):771-6.
17. AREDS2-HOME Study Research Group, Chew EY, Clemons TE, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014 Feb;121(2):535-44.
18. Ying G, Huang J, Maguire M, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013 Jan;120(1):122-9.

