 |
The earliest antibiotics, such as bacitracin, attack bacterial cell walls. This allows for an influx of fluid, ultimately causing cellular lysis. While this strategy is successful against gram-positive organisms, it is not effective against gram-negative bacteria because they have complex outer cell membranes that encapsulate their cell walls.
By contrast, agents such as polymyxin B destroy cell membranes. This makes them ideal for eradicating gram-negative bacteria, but they are virtually ineffective against gram-positive organisms, which lack a cell membrane. This dilemma eventually led to the development of Polysporin (bacitracin zinc and polymyxin B, Monarch), one of the earliest broad-spectrum drugs.
Other early antibiotics, such as sulfacetamide, selectively compete for and inhibit crucial elements of bacterial metabolism. Sulfacetamide selectively binds para-aminobenzoic acid (PABA), a key component in the metabolism cycle of folic acid. When bacteria cannot access this crucial element, their cellular processes stagnate, and they fail to reproduce.
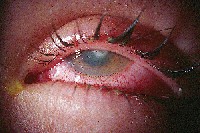 |
| Antibiotics have come a long way over the years in treating corneal ulcers. |
The newest topical broad-spectrum agents, the fluoroquinolones, inhibit DNA gyrase and topoisomerase IV. These enzymes play a crucial role in bacterial cell division. Early-generation fluoroquinolones, such as levofloxacin, ciprofloxacin and ofloxacin, target only one enzyme at a time. However, the fourth-generation fluoroquinolonesZymar (gatifloxacin 0.3%, Allergan) and Vigamox (moxifloxacin 0.5%, Alcon)simultaneously target both enzymes.
The Need for New Antibiotics
We are always looking for new antibiotics because as bacteria evolve and adapt to their environment, they develop immunity to antibiotics. Nearly every ophthalmic antibiotic has been met with some resistance.
Sulfacetamide began to show resistance to gram-positive bacteria 20 years ago. Gentamicin and tobramycin, deemed the big gun antibiotics in the mid-to-late 1980s, now encounter resistance rates as high as 40% among some bacterial strains.1-3 Even the early-generation fluoroquinolones face resistance to some common bacteria, particularly gram-positive organisms.4,5
Another reason we look for new antibiotics: toxicity. For example, sulfacetamide and neomycin are notorious for causing hypersensitivity reactions. Gentamicin is well known for its corneal toxicity, particularly when used for prolonged periods. Ciprofloxacin is an extremely potent antibiotic, but it demonstrates poor solubility under normal circumstances. Hence, it must be formulated at a pH of 4.5 (roughly the equivalent of tomato juice).
These agents also cause signifi-cant epithelial disruption, which can delay healing. So, newer agents sometimes gain favor because they are less toxic and require less frequent dosing than older agents.
Antibiotics in Development
Four antibiotics are currently in development:
Iquix (levofloxacin 1.5%, Santen Pharmaceutical Co.). The FDA approved this third-generation fluoroquinolone in March 2004 for the treatment of bacterial corneal ulcers. As of press time, Santen has not released the drug. We suspect that this is because many practitioners already use the fourth-generation fluoroquinolones for treating bacterial corneal ulcers. Although Vigamox and Zymar are specifically indicated for treating bacterial conjunctivitis, numerous studies have shown their efficacy at treating bacterial keratitis as well.6-8
AzaSite (azithromycin 1%, InSite Vision Inc.). This macrolide antibiotic is the first topical formulation of azithromycin for ophthalmic use. InSite Vision claims that by combining azithromycin with DuraSite, a patented drug-delivery system, this medication offers diminished dosing frequency with increased efficacy.
AzaSite is currently in Phase III human clinical trials to determine its safety and efficacy in treating acute bacterial conjunctivitis. In June 2005, an InSite press release referenced a study in which AzaSite was found to be as effective as a fourth-generation fluoroquinolone against Pseudomonas aeruginosa keratitis in a rabbit model. InSite anticipates filing a New Drug Application with the FDA sometime in 2005.
ISV-403 (Bausch & Lomb). This new fourth-generation fluoroquinolone, currently in the early phases of clinical trials, combines the aforementioned topical antibiotic with DuraSite to offer diminished dosing frequency. Preclinical studies indicate that ISV-403 is effective against bacteria resistant to second- and third-generation fluoroquinolones, B&L says.
Trovafloxacin. Pfizer introduced this fourth-generation fluoroquinolone to the U.S. market as Trovan in 1998. In June 1999, however, the FDA issued a public health advisory in which it warned doctors about severe liver toxicity associated with the drug and recommended its use only for severe cases of infection. The drug was discontinued in 2003. Nonetheless, topical trovafloxacin 0.5% was shown to effectively eradicate both gram-positive and gram-negative bacteria in a rabbit keratitis model.9 The drug is also reportedly being studied for the treatment of bacterial endophthalmitis through intravitreal injection.
Ocular infections that become unmanageable can rapidly result in functional impairment. So, we need to understand the methods by which currently available antibiotics work, why new antibiotics are needed and what new antibiotics can offer to our patients.
Drs. Kabat and Sowka are members of Alcons speakers alliance. They have no financial interest in any of the products mentioned.
1. Schmitz FJ, Verhoef J, Fluit AC. Prevalence of aminoglycoside resistance in 20 European university hospitals participating in the European SENTRY Antimicrobial Surveillance Programme. Eur J Clin Microbiol 1999 Jun;18(6):414-21.
2. Pfaller MA, Jones RN, Doern GV, et al. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997). Antimicrob Agents Chemother 1998 Jul;42(7):1762-70.
3. Vakulenko SB, Mobashery S. Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev 2003 Jul;16(3):430-50.
4. Block SL, Hedrick J, Tyler R, et al. Increasing bacterial resistance in pediatric acute conjunctivitis. Antimicrob Agents Chemother 2000; Jun;44(6):1650-4.
5. Ritterband DC, Farquhar A, Shah M, et al. Are second generation fluoroquinolone antibiotics losing their competitive edge in precataract surgery prophylaxis? Invest Ophthalmol Vis Sci 2002;43: E-Abstract 1580.
6. Aliprandis ET, Ciralsky J, Lai H, Herling I, Katz HR. Comparative efficacy of topical moxifloxacin versus ciprofloxacin and vancomycin in the treatment of P. aeroginosa and ciprofloxacin-resistant MRSA keratitis in rabbits. Cornea 2005;24(2):201-5.
7. Katz H. Successful treatment of a Pseudomonas aeruginosa corneal ulcer in a human patient with moxifloxacin 0.5%. Presented at the 22nd Congress of the European Society of Cataract and Refractive Surgeons, Paris, September 2004.
8. Caldern D, Kabat AG, Schinas Z, Corella CW. Successful management of a central bacterial corneal ulcer with topical moxifloxacin and the importance of concurrent corticosteroid use. Poster presented at the 108th Congress of the American Optometric Association, Dallas, June 2005.
9. Barequet IS, Denton P, Osterhout GJ, et al. Treatment of experimental bacterial keratitis with topical trovafloxacin. Arch Ophthalmol 2004;122(1):65-9.

