 |
Many patients have told me their red eyes have kept them from going out with friends or family, and they were tired of being asked if they were crying, drinking, sleep-deprived or on drugs. One patient told me that she had more than 35 interviews for a job and felt she was well qualified, but that her chronic red eyes prevented her from being hired (whether it was affecting her confidence or the interviewer’s perception). I had her try a new over-the-counter red eye reducer—one I’ve been involved with as a consultant for Bausch + Lomb—and she reported being offered a job after her very next interview. Although just one anecdotal experience with a plethora of underlying factors at play, it exemplifies the significant personal and social connotations of chronic red eyes for our patients.
The new red eye reducer I used was Lumify (brimonidine tartrate ophthalmic solution 0.025%, Bausch + Lomb), which could have significant implications for many of your patients.
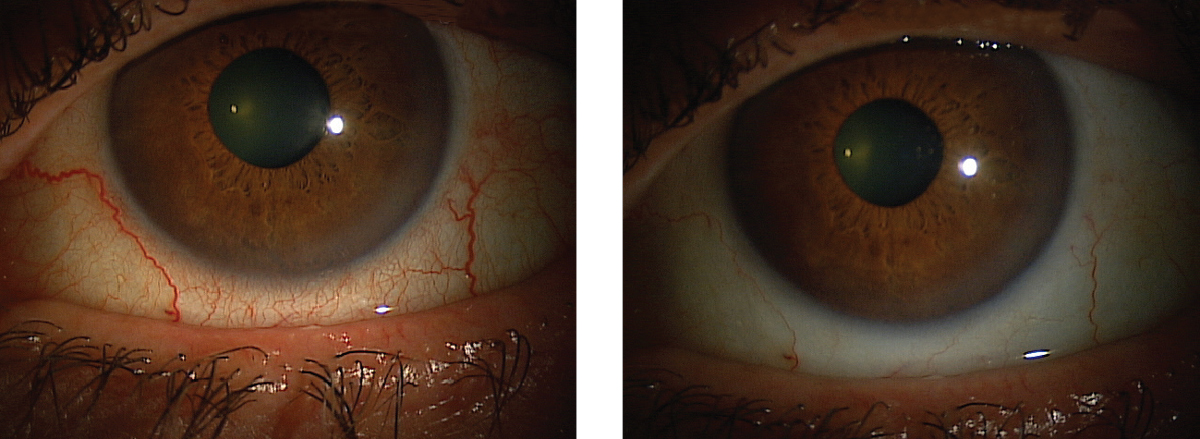 |
| This patient’s red eyes cleared up after instilling Lumify, and they can expect the effects to last six to eight hours. Click images to enlarge. |
Something New
Recently FDA-approved for the relief of ocular redness due to minor eye irritations, Lumify is a low-dose (0.025%) brimonidine tartrate and is a highly selected α-2 adrenergic receptor (AR) agonist. It is approved for ages five and older, works within 30 to 60 seconds and lasts six to eight hours with no known rebound hyperemia or tachyphylaxis. As an aside, a non-ocular formulation exists, 0.33% brimonidine gel, for the treatment of persistent facial erythema secondary to rosacea.1
We’ve had almost 20 years of experience with high doses of brimonidine tartrate when managing glaucoma patients. It is the active ingredient in Alphagan P (Allergan), for example. However, the concentration of Alphagan P is 0.1% and generic forms are 0.2%. The higher dose is associated with hyperemia and allergic dermatitis or ocular allergy; very low doses, however, are known to have a whitening or blanching effect.2-4 Researchers realized reducing the concentration four- or eight-fold avoids IOP changes while enhancing the whitening effect.5
Why It’s Different
Red eye reducers on the market thus far affect the α-1 receptors alone or α-1 and α-2 receptors non-selectively. Visine (tetrahydrozoline, Johnson & Johnson) is an example of a selective α-1 AR agonist, while mixed α-1 AR and α-2 AR agonists include naphazoline—available as ClearEyes (Prestige Brands), Opcon-A (Bausch + Lomb) and Naphcon-A (Alcon)—and oxymetazoline, marketed as Visine LR (Johnson & Johnson) and OcuClear (Elder Pharmaceuticals).
Long-term use of these redness reducers is limited due to rebound hyperemia, tachyphylaxis, pupil dilation, corneal toxicity and systemic side effects such as dizziness.6,7 Research shows ocular toxicity due to extended use can be so severe as to mimic ocular cicatricial pemphigoid.8 The rebound hyperemia and tachyphylaxis is due to the preponderance of α-1 ARs in arteries.8 Their presence results in constriction of arterial blood flow and oxygen delivery. The body’s response is to initiate down-regulation of α-1 ARs, resulting in tachyphylaxis. The vasoconstrictor-induced tissue becomes ischemic and the body’s response is vasodilation—hence the rebound redness.9
In contrast, Lumify is a selective α-2 AR agonist, with minimal action at the α-1 AR site. This, provides preferential venule constriction rather than affecting oxygen flow through the arteries, eliminating the resultant ischemia.10 Constriction, which affects the veins—a significant anatomical target, given the concentration of vortex veins and capillary bed volume—results in an increased whitening effect without the risks of tachyphylaxis or rebound hyperemia.10 Furthermore, since the drug lasts six to eight hours, it can typically be dosed once or twice a day, further decreasing the risk of toxicity and medicamentosa.11 In FDA Phase II and III trials, neither tachyphylaxis or rebound hyperemia were noted.12
The ocular adverse events, such as burning on instillation, were minimal and mild and resolved spontaneously. Patients reported the drop as ‘very comfortable,’ and researchers detected no systemic safety signals in either the efficacy studies and or the safety study of pediatric, adult and geriatric subjects.11,12
Clinical Outlook
So far, I have found this product helpful for patients with conjunctival redness due to ocular irritation such as dry eyes, allergies, rosacea, meibomian gland dysfunction and especially for chronic hyperemia patients.
In this era of information-on-demand for patients, we must always be aware of new therapeutics such as low-dose brimonidine. Patients will see this drop on the shelf next to their current eye whitener, and may come to you seeking advice and information about its effects.
We now have a tool at our disposable to provide these patients whiter eyes without the complications and systemic risks of other vasoconstrictors. This provides yet another opportunity to increase your patients’ confidence in your management of their ocular condition and, even more important, increase their own confidence in social circumstances.
1. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-6. |

