 |
Since their introduction in 1996, prostaglandin analogs (PGAs) have been the mainstay of glaucoma treatment due to their efficacy, once-daily dosing and limited adverse effect profile.1 The aim of all medical and surgical glaucoma interventions is to reduce intraocular pressure (IOP), to date the only modifiable factor. Since most eye care practitioners gravitate towards the use of these drugs as first-line glaucoma treatment—and a new one has just arrived—it’s important to understand exactly what a PGA is and how it works to lower IOP.
What is a Prostaglandin?
Pro-inflammatory molecules that bind to receptors throughout the entire body, including ocular structures, prostaglandins (PGs) elicit several effects. There are approximately nine types of PGs in the body, but only the PGF2 subtypes are currently targeted in glaucoma treatment because they are located directly on aqueous outflow structures and activation of this specific receptor affects aqueous humor dynamics.2
PGs are generally produced through the arachidonic acid pathway, in which the latter substance is released from the plasma membrane and metabolized by the cyclooxygenase enzymes. As such, they play an important role in immune system regulation and inflammation.1 Additionally, PGs function in the constriction and relaxation of smooth muscle, adipocyte differentiation and remodeling of the extracellular matrices found throughout the body.3-5 It is the latter—a PG’s role in extracellular matrix remodeling—that yields its primary mechanism in increasing aqueous outflow to reduce IOP in glaucoma patients. PGs also elicit their known effect on smooth muscle by binding to the PG receptors located in the ciliary muscle, causing relaxation and, subsequently, increased aqueous outflow.1,6
Also, at the site of the ciliary muscle, the iris root and sclera, PGs induce matrix metalloproteinase (MMP) expression, a critical component in connective tissue remodeling. As a result, the extracellular matrix is modified to reduce outflow resistance and lower IOP. More recent histological studies support a similar mechanism of tissue remodeling at the level of Schlemm’s canal, providing evidence that PGAs act on the conventional pathway as well, although to a lesser degree.3,6
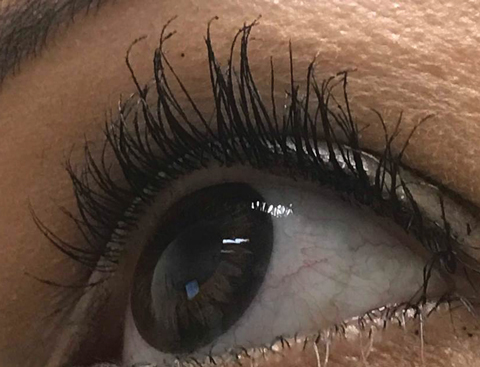 |
| Trichomegaly resulting from prolonged use of PGA for cosmesis. |
PGA Tour
Topical drugs that mimic the function of naturally occurring PGs are called prostaglandin analogs, given their pharmacodynamic similarities to some PG-mediated processes. PGAs are stand-ins for PGs, but not identical in form or function.
Traditionally, eye doctors have relied on four topical PGAs: latanoprost, bimatoprost, travoprost and preservative-free tafluprost ophthalmic solutions.2 While all these agents are classified as prodrugs of PGF2, only latanoprost, travoprost, and tafluprost are prostanoids. This means that following topical instillation, enzymes on the corneal surface hydrolyze the drug into a biologically active form. Bimatoprost, on the other hand, is classified as a prostamide. This difference in its chemical makeup has led to studies questioning whether this is in fact a true prodrug of prostaglandins, as the drug remains mostly unchanged following topical administration.1
In November 2017, a new PGA combination drug gained FDA approval. Vyzulta (latanoprostene bunod ophthalmic solution 0.24%, Valeant Pharmaceuticals) contains a PGA as well as a nitric oxide (NO) metabolite. Besides the mechanisms of PGAs described above, NO has been added to further increase aqueous outflow through the conventional pathway, by directly working on the trabecular meshwork and causing relaxation and outflow. This dual-action drug has been shown more efficacious than a PGA alone in clinical trials, with a mean decrease of 9mm Hg after 28 days of use in the Vyzulta group vs. a mean decrease of 7.77mm Hg with latanoprost 0.005% alone.7 Latanoprostene bunod is nearly identical to the molecular structure of latanoprost, except that it contains a terminal NO group.8 Because of this similarity, the side effects are the same as the current topical PGAs on the market.9
Review of Outflow PathwaysIOP is maintained through a balance of aqueous humor production and drainage. In most glaucoma cases, it is impaired drainage that results in elevated IOP and subsequent optic nerve damage. Aqueous drainage from the anterior chamber is permissible through two mechanisms: the conventional outflow pathway and the unconventional pathway.1 The conventional outflow pathway, responsible for 60% to 80% of aqueous drainage, mainly involves filtration through the trabecular meshwork and Schlemm’s canal, which results in the aqueous ultimately exiting through the episcleral venous system. In contrast, the remaining 20% to 40% of aqueous that is produced drains through the unconventional, or uveoscleral, pathway by diffusing through the interstitial spaces of the ciliary muscle and ultimately the suprachoroidal space.1 These pathways often become resistant to drainage in glaucoma patients, making them key targets for drug therapies. While PGAs have been thought to work mainly on the unconventional pathway, more recent evidence supports their role in increasing outflow through the conventional pathway as well.1,2 |
Side Effects of Treatment
Given the integral role PGs play in the initiation of the acute inflammatory pathway, the most common side effect of topical PGAs is conjunctival hyperemia or inflammation.5 Clinicians should exercise caution when using topical PGAs in patients with inflammatory ocular conditions such as postoperative cystoid macular edema or uveitis.4,10 Researchers looked at PG levels in dry eye disease and concluded that ocular injection, pain and discomfort from dryness (as well as PGA use) may be due to significantly elevated levels of PGs on the ocular surface, which correlated with patient symptoms.11
PGs also have an effect on adipogenesis—the differentiation of cells into adipocytes, or fat cells. Studies show that PGAs inhibit this process, and topical use decreased dermatochalasis and cause deepening of the upper lid sulcus.4,10
The synthesis of MMPs and the extracellular matrix remodeling effect of PGAs also occurs at the level of the cornea, with one study concluding that the use of latanoprost resulted in an increase in corneal hysteresis.3
Cosmetically, the use of topical PGAs results in iris hyperpigmentation and trichomegaly, or eyelash growth. The mechanism of induced iris hyperpigmentation is not well understood, but appears to be secondary to PG stimulation of iris melanocytes resulting in melanin production and melanocyte migration. These effects are often experienced following three to six months of treatment and most commonly affect hazel-colored irides.12
Trichomegaly occurs through PG stimulation of melanocytes in the hair follicle, as well as stimulation of follicles into the anagen, or active growth, phase.13 The effect of these induced processes is greater lash frequency, thickness and length. This mechanism forms the basis of the FDA approval of a topical PGA for eyelash lengthening.14
PGAs in Practice
Without PGAs, our ability to control IOP would be markedly reduced. While other glaucoma drug classes serve us well, a PGA’s ability to improve aqueous outflow gives clinicians one more lever to pull in managing the delicate balance of forces that govern IOP—and, by extension, glaucoma progression.
Natural prostaglandins perform several functions; our understanding of these illuminates the mechanisms of IOP lowering as well as the side effects and contraindications we discuss with our patients. Of the nine PG subtypes, only one is currently targeted in glaucoma. Other receptors may one day serve as potential treatment sites for patients who require additional therapy.
1. Winkler NS, Fautsch MP. Effects of prostaglandin analogues on aqueous humor outflow pathways. J Ocular Pharmacol Thera. 2014;30(11):102-9. |

