Glaucoma is the second leading cause of blindness worldwide, projected to affect nearly 80 million people by 2020.1 While several different forms of treatment are available for this debilitating disease, all modalities share the same goal: to preserve vision by lowering intraocular pressure (IOP). The remarkable advancements in surgical techniques, particularly in the areas of glaucoma laser procedures and minimally invasive surgeries, means glaucoma specialists increasingly rely upon the primary care optometrist for pre- and postoperative support.
This article reviews important concepts to better equip optometrists in appropriately managing patients who require glaucoma surgery. We will discuss considerations for surgical intervention, introduce common and emerging surgical procedures and provide expectations of postoperative management. The objective: solidify the optometrist’s understanding to better comanage post-op glaucoma patients.
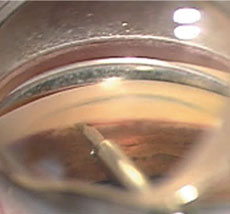 | 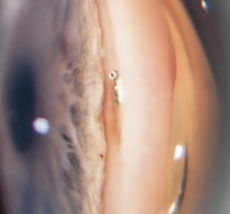 |
| At left, an intraoperative photo of the Trabectome employing electrocautery and aspiration to remove tissue of the trabecular meshwork within the anterior chamber angle. At right, a postoperative gonioscopic photo shows the iStent device through the trabecular meshwork into Schlemm’s canal. Used during the time of cataract surgery, the iStent requires no additional port incisions to be implanted. | |
Surgical Indications
Once considered a last resort, surgery is increasingly viable, even preferable, earlier in the course of the disease. Here are a few reasons to consider surgery for your glaucoma patients.
• Therapeutic failure. Reaching an appropriate target IOP for a glaucoma patient may be unobtainable with medical therapy alone, especially if the patient first presents with more advanced disease. The Ocular Hypertension Treatment Study (OHTS) determined that 39% of eyes required two or more medications to achieve a 20% reduction from baseline IOP.2 Since most patients are initially treated with prostaglandin analogs, practitioners conventionally use β-blockers, α-agonists, carbonic anhydrase inhibitors or a combination of these to further lower IOP. However, most of these second-line drugs show reduced efficacy when used as additive treatments compared with their use as monotherapies.2
Bottom line: If target IOP is not achieved, or your patient is showing progressive structural or visual field loss on maximum drug therapy, surgical intervention is required.
• Poor compliance. Achieving medication compliance can be a major hurdle for glaucoma patients. Noncompliance with glaucoma regimens is reported to be quite high, ranging anywhere from 25% to 80%.3 In addition, as the dosing schedule becomes more complex, compliance wanes. In an effort to determine how compliance is affected when an additional medication is added to the regimen, researchers investigated the refill intervals of 4,930 patients using latanoprost before and after adding a second medication. Once the second drug was added, these patients refilled their latanoprost less frequently compared with monotherapy alone.4
From a pragmatic standpoint, a glaucomatous eye can be treated with three different drug classes, using as few as three drops per day if a prostaglandin and one of the combination drugs available on the market are used (i.e., brimonidine-timolol, dorzolamide-timolol and brinzolamide-brimonidine). However, adding a fourth drug can complicate the dosing schedule too much, affecting compliance.
Bottom line: If your patient has a protracted history of noncompliance with drops or if you face the prospect of adding a fourth drug class, it may be time to consider surgery.
• Expense. The cost of medications can complicate, and often limit, how we treat our patients. With glaucoma affecting more than three million Americans—overwhelmingly in the senior citizen cohort, who mostly rely on a fixed income—the United States spends an estimated $2.9 billion annually on glaucoma drugs. The annual cost of care per patient is projected to range from $623 to $2,511, depending on the severity of the disease.5 Pharmaceutical treatments for glaucoma can be a serious financial burden to our patients—drug cost is among the top barriers to medication adherence.6 Adding to the problem, several glaucoma drugs remain unavailable as generics. Insurance plans may require evidence of therapeutic failure or intolerance to cheaper medications before approving coverage for brand-name drugs.
Bottom line: If your patient is having difficulty affording their glaucoma medications, surgical options may provide a long-term cost savings, depending on their medical insurance coverage.
• Adverse effects. Intolerance to topical medications can limit our ability to medically manage glaucoma patients. Optometrists should always be mindful of the side effects associated with the staple glaucoma drugs, such as the cardio and pulmonary effects of β-blockers and the cosmetic changes associated with prostaglandins that include pigmentary darkening around the eyelids, eyelash growth, iris color darkening and periorbital fat loss.
Carbonic anhydrase inhibitors are sulfa drugs and, though they differ in composition from sulfonamide antimicrobials, may cause adverse effects in patients with sulfa allergies. Researchers found that up to 25.7% of patients using 0.2% brimonidine develop an allergic conjunctivitis in response to their drops.7 Furthermore, repeated exposure to high amounts of preservatives, such as benzalkonium chloride (BAK), has been associated with ocular surface changes, especially for patients with pre-existing ocular surface disease.8
Bottom line: If patients are intolerant to one or more glaucoma medications, surgery to lower IOP may be necessary.
• Narrow angles. It’s essential to assess the iridocorneal angle of all glaucoma patients with gonioscopy. A recent epidemiological study estimates angle closure glaucoma affects 16 million people worldwide, rendering up to 25% of these patients bilaterally blind.10 If left untreated, an angle-closure attack in one eye means a 40% to 80% chance of developing an attack in the fellow eye in five to 10 years.9
Bottom line: If your patient is at risk for angle closure or has developed one, surgical intervention is indicated.
Minimally Invasive Surgeries
After decades of stagnation, the options for surgical management have diversified in recent years. Minimally invasive glaucoma surgery (MIGS) options are the hot topic in interventional glaucoma, as these less effective but more patient-friendly alternatives find an appropriate place in the glaucoma management hierarchy.
MIGS Key PointsIndications: MIGS procedures are typically used in patients with mild to moderate glaucoma who are candidates for cataract surgery. The Post-Op Evaluation: Since many MIGS procedures are performed in conjunction with cataract surgery, the post-surgical course is often quite similar to that of cataract surgery alone. Patients should continue their regular glaucoma medications immediately after surgery as the IOP reduction from the procedure does not fully manifest for six to eight weeks. Pros: The hallmark of MIGS is the high safety profiles. These procedures possess minimal risk of adverse effects and complications. They may be a safe means of reducing or eliminating glaucoma medications in many of our patients. Cons: Since the IOP-reducing effect with MIGS is not as substantial as the more invasive glaucoma surgeries, such as trabeculectomy and ab-externo glaucoma drainage implants, MIGS is not indicated for patients with severe disease requiring very low (often in the single-digit) IOP profiles. |
The more invasive surgeries, such as trabeculectomy and ab-externo glaucoma drainage implants, are associated with significant risk of postoperative complications.10 In contrast, MIGS procedures are performed through small incisions, often during cataract surgery using the same incision, and demonstrate excellent safety profiles.11 Optometrists will find themselves referring for and comanaging these procedures more frequently in the coming years; therefore, it is essential to be familiar with these options. Here, we cover a few of the more popular MIGS procedures.
• Trabectome (Neomedix). FDA approved in 2004, this procedure is performed with a handheld device inserted into the anterior chamber through a small corneal incision. The Trabectome is positioned through the trabecular meshwork (TM) into Schlemm’s canal, where it employs electro-cautery and aspiration functions to remove strips of tissue within the angle. This mechanism reduces resistance and aids aqueous drainage.12 Surgeons can use the Trabectome in isolation or during cataract surgery. It should be noted that adding the Trabectome to cataract surgery has not been reported to increase complications compared to cataract surgery alone.12,13
Does it work? In 2015, researchers followed 82 treated eyes and found a 23% reduction from pre-treatment IOP at the two-year mark. Other studies estimate the IOP reduction can range from 16% to 44% during the one- to two-year period after treatment.13
Although the postoperative medication schedule can vary by surgeon, patients are typically prescribed topical fluoroquinolones QID for one week and topical steroids QID tapered over the next one to two months. Additionally, pilocarpine 1% to 2% is applied BID to QID and tapered along with the steroid drops; this prevents peripheral anterior synechiae and concomitantly lowers IOP.12-14
The Trabectome procedure comes with the risk for postoperative IOP spike and hyphema; however, these adverse events are rare and usually resolve quickly. Preoperative glaucoma medications should be continued immediately following the Trabectome procedure and adjusted appropriately as the IOP profile stabilizes in six to eight weeks. The preoperative glaucoma medications will not alter the probability of a post-op IOP spike, but will reduce its severity if it occurs.
• iStent (Glaukos). In 2012, the iStent was FDA approved for use in combination with cataract surgery. The heparin-coated titanium device measures 1mm by 0.3mm, making it the smallest FDA-approved device for implantation in the human body. During cataract surgery, the device is implanted into Schlemm’s canal where it remains permanently to improve aqueous outflow. The iStent is non-magnetic and thus compatible with magnetic resonance imaging (MRI).15
Does it work? In December 2015, researchers published exciting results after following 41 eyes implanted with a single iStent over three years. The average preoperative IOP for the subjects was 24.1mm Hg; on average, the patients were taking 1.8 glaucoma medications. At the three-year post-op interval, the average IOP was 14.9mm Hg with medications being eliminated in 74% of eyes.16
However, not all studies have found such optimistic results. A meta-analysis published in July 2015, which evaluated 37 studies reporting on 2,495 patients, concluded that cataract surgery alone reduced IOP by 4% while the combined phacoemulsification/iStent procedure reduced IOP by 9%.17
A second generation of the iStent is currently available in Europe. With a modified design, it comes pre-loaded with two iStents. When researchers conducted a meta-analysis of cases involving two istents implanted in a single eye, they showed IOP was reduced by 27% from baseline.17 With the possibility of approval in the United States for the dual iStent design, there is hope for even greater IOP reduction for American glaucoma patients.
It is recommended to wait six to eight weeks before observing the new postoperative IOP state. Patients should therefore continue their glaucoma medications immediately after surgery, and clinicians can discontinue meds as the IOP improves. Similar to the postoperative course in cataract surgery, patients are prescribed a topical fluoroquinolone QID for seven days. Steroids starting at QID after surgery should be tapered over the next one to two months, and a topical NSAID QID should be given to supplement the steroid. Some clinicians may choose to taper the steroids more quickly than with the average postoperative cataract patient, as glaucoma patients can be more vulnerable to an IOP steroid response.
• Cypass (Transcend Medical/ Alcon). Cypass is the newest MIGS device to hit the market, having just recently achieved its FDA approval for use in conjunction with cataract surgery on July 29th, 2016. Unique from the other devices we have discussed, Cypass targets the suprachoroidal space to increase aqueous outflow.
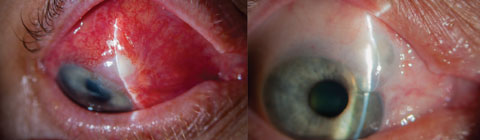 |
| Blebitis (left) and over-filtration of a bleb (right) are among complications associated with more invasive surgeries such as trabeculectomy. MIGS, in contrast, provide better safety profiles, fewer complications and faster recovery times. Photos: Trey Sullins, OD. Click image to enlarge. |
The device is a tube shunt made of polyamide material that measures 6.35mm long and a mere 510 microns in diameter. It is carefully placed in the angle between the ciliary body and the sclera and terminates in the suprachoroidal space. The Cypass has openings at each end and micro-holes along its exterior to allow aqueous to flow into the shunt from the anterior chamber and exit the shunt into the suprachoroidal space.
Does it work? One study in particular is responsible for the device’s FDA approval. The COMPASS Trial, a multicenter, randomized clinical trial, was published in August 2016.18 COMPASS trial followed 505 subjects. One hundred and thirty-one subjects were randomized into the control group to receive phacoemulsification alone; the remaining 347 received phacoemulsification and Cypass placement. All subjects had primary open-angle glaucoma with entering unmedicated IOP ranging from 21mm Hg to 33mm Hg. The study found that mean IOP was reduced by 7.4mm Hg for the Cypass group and 5.4mm Hg for the control group at the two-year postoperative checkpoint.18 Although the difference between groups was only 2.0mm Hg, this was statistically significant enough for the study to conclude that Cypass provided long-term IOP reduction.18
At the two-year mark, medications were completely eliminated in 85% of the Cypass group, vs. 59% of the control.18 No severe or visually threatening events occurred throughout the study. The mild adverse events reported were iritis, corneal edema, hypotony and IOP elevation. However, these events were quite rare.
The COMPASS trial followed patients in the immediate post-op period at day one, week one, month one and month three. Patients were put on topical antibiotic drops for one week, topical NSAID drops for three weeks, and topical steroid drops, tapered over one month. During the clinical trial, patients were left off of their glaucoma medications postoperatively; they were restarted on glaucoma medications on a case-by-case basis if IOP remained elevated for two consecutive visits.
• Endoscopic cyclophotocoagulation (ECP) aims to lower IOP by diminishing production of the aqueous by the epithelium of the ciliary processes. ECP is performed in conjunction with cataract surgery and uses a curved endoscopic laser probe. The surgeon applies laser energy to between 270 and 360 degrees of the ciliary processes, thereby reducing aqueous production.
Does it work? The results vary between studies. In 2016, researchers followed a cohort of 91 eyes for one year and report an IOP reduction of 19% from baseline.19 Two years prior, scientists studied 80 eyes over the course of two years and report an average IOP reduction of only 10%.20 Then, in 2015 another set of researchers published a retrospective study of 261 eyes with results three years postoperatively; their research shows a 14.5% average reduction in IOP. 21
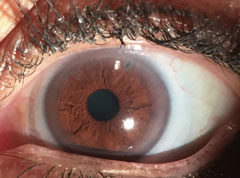 |
| External photo of the patient’s eye after the laser peripheral iridotomy. |
In each of these studies, no increase exists in the rate of complications for combined ECP and phacoemulsification compared with phacoemulsification alone. Although the results do not always yield a dramatic reduction in IOP, the Early Manifest Glaucoma Trial shows the risk of progression decreases by 10% for every 1mm Hg reduction of IOP.22 With minimal risks involved, ECP is a procedure worth considering for glaucoma patients concomitantly in need of cataract surgery.
Glaucoma Laser Procedures
These mainstays are familiar to practicing optometrists and remain viable in our long-term glaucoma management efforts.
• Laser trabeculoplasty using an argon laser (ALT) was first introduced in 1979. In this procedure, thermal energy is applied to the trabecular meshwork (TM), which induces contracture of the affected tissue. These focal alterations allow adjacent areas of the TM to expand, decreasing outflow resistance.23 Due to its limited repeatability over time, ALT’s role has not historically been found to be superior to medical therapy.
Since its approval in 2001, selective laser trabeculoplasty (SLT) has taken over as the preferred means of laser trabeculoplasty. SLT uses a frequency-doubled (532nm), Q-switched Nd:YAG laser to deliver laser pulses over 180 to 360 degrees of TM. These pulses selectively target the pigmented cells of the TM, which increase photolysis and cytokinetic activity, causing a healthy restructuring of the TM.
The limited structural damage seen in SLT allows for SLT to be repeated in the same eye if efficacy wanes or if further IOP reduction is needed. This has ultimately allowed clinicians to use this procedure as an initial treatment option in mild to moderate cases of primary and secondary open-angle glaucoma.23 Selective laser trabeculoplasty’s success rate
—defined as 20% IOP reduction—was found to be between 55% and 82% in certain studies.23 SLT typically shows greater efficacy in patients who have higher pre-treatment IOP values.
Alpha-agonist drops, such as brimonidine or apraclonidine, are usually instilled immediately before and after SLT. IOP should be checked one hour after SLT is performed to rule out a transient postoperative spike, which has been reported to occur in 4.5% to 27% of patients.24 Patients should continue their glaucoma regimen; topical anti-inflammatory drops are typically applied for five to seven days.
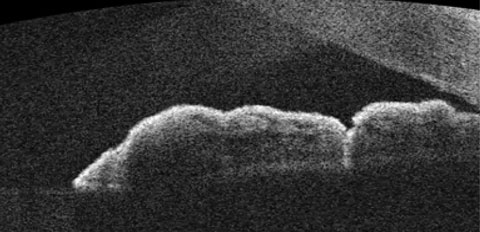 |
| OCT of the angle following LPI treatment. Observe the more direct channel that is created, which relieves the risk of angle-closure. |
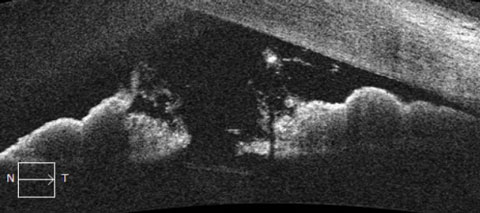 |
| OCT images of the anterior segment demonstrating relative pupillary block. Note the apposition of the posterior pigmented epithelium of the iris with the anterior lens capsule. The obstruction of aqueous flow causes a fixed, mid-dilated pupil and bowing of the peripheral iris at the angle. |
In a recent study that compared artificial tears, prednisolone acetate and ketorolac, each dosed QID following SLT, there was no significant difference in the IOP-lowering outcome or effect on failure rates of the procedure after one year.26 It is common for patients to report varying levels of postoperative discomfort, and up to 50% can present with postoperative anterior chamber reactions at the standard one-week followup. Therefore, the post-op management should focus not only on evaluating IOP, but also maximizing patient comfort with appropriate anti-inflammatory drops.23-25 Patients should remain on their glaucoma medications after the procedure and be re-evaluated at one and three months. It’s recommended to wait at least six to eight weeks before adjusting a patient’s glaucoma medications, as SLT reaches its steady-state at this time.
• Laser peripheral iridotomy (LPI) is indicated for the treatment of (1) angle-closure glaucoma (ACG) associated with relative or absolute pupillary block and (2) prophylactic management of patients with narrow anterior chamber angles who may be at risk for ACG. This procedure uses an argon or Nd:YAG laser to create a full-thickness opening of the peripheral iris. This allows the aqueous humor to bypass its normal course through the pupil, and gives the fluid a direct pathway from the posterior chamber into the anterior chamber and ultimately to the trabecular meshwork. It effectively eliminates iridolenticular obstruction to aqueous flow.
IOP should always be checked approximately one hour after the applied laser to rule out a transient IOP spike. Patients should be given a topical steroid QID for five to seven days and should continue any glaucoma medications they are taking. A one-week postoperative visit should entail an IOP evaluation, a check for patency of the iridotomy with direct and retroillumination, and should address the presence of any post-procedural inflammation. Patients should be re-examined at one month to ensure stable IOP; a further evaluation of the anterior chamber angle should be conducted via gonioscopy and, if available, anterior segment optical coherence tomography (AS-OCT). If a dilated fundus exam is indicated, post-dilation IOP should be documented to help provide evidence of a properly functioning LPI.
Although the risk is low, there are potential adverse effects associated with laser iridotomies. A marked increase in IOP and mild iritis following the procedure may occur in up to 30% to 35% of cases.26 Intraocular inflammation is usually observed within the first 24 hours and resolves either spontaneously or with topical anti-inflammatory drops. Structural damage to the cornea and lens is possible, along with the later development of peripheral anterior synechiae and hyphema. Less common, but more visually threatening, complications such as retinal and choroidal detachments, focal retinal burns and macular edema can occur.
Summary
Although most of our glaucoma patients can be medically managed using topical IOP-lowering drugs, MIGS and glaucoma laser procedures offer numerous benefits for those battling progressive disease, medication cost, difficulty with compliance or intolerance to eye drops. These procedures will become ever more popular for our mild to moderate stage glaucoma patients in the years to come. Familiarity with the key principles of appropriate comanagement will help you provide the best care to patients who have undergone these surgical techniques.
Dr. Van Alstine practices at the WJB Dorn VA Medical Center in Columbia, SC. He also serves as the president of the South Carolina chapter of the American Academy of Optometry.
Dr. Caruso practices within the Ralph H. Johnson VA Medical Center at the outpatient clinic in Myrtle Beach, SC.
|
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006 Mar;90(3):262–7. 2. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002 Jun;120(6):701-13; discussion 829-30. 3. Olthoff CM, Schouten JS, Van De Borne BW, et al. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: An evidence-based review. Ophthalmology. 2005;112:953-61. 4. Robin A, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy? Ophthalmology. 2005;112:863-8. 5. Chan P, Li EY, Tham CC. Cost-effectiveness in the treatment of glaucoma. US Ophthalmic Review. 2014;7(2):131–6. 6. Newman-Casey PA, Robin AL, Blachley T, et al. Most common barriers to glaucoma medication adherence: A Cross-Sectional Survey. Ophthalmology. 2015;122(7):1308-16. 7. Blondeau P, Rousseau JA. Allergic reactions to brimonidine in patients treated for glaucoma. Can J Ophthalmol. 2002 Feb;37(1):21-6. 8. Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. Arch Ophthalmol. 1994;112:1437-45. 9. Cumba RJ, Nagi KS, Bell NP, et al. Clinical outcomes of peripheral iridotomy in patients with the spectrum of chronic primary angle closure. ISRN Ophthalmology. 2013 Jun 26;2013:828972. 10. Gedde S, Herndon L, Brandt J, et al. Tube Versus Trabeculectomy Study Group. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804-14. 11. Wellik SR, Dale EA. A review of the iStent trabecular micro-bypass stent: safety and efficacy. Clinical Ophthalmology. 2015 April;2015(9):677-84. 12. Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clinical Ophthalmology. 2016;10:189-206. 13. Mizoguchi T, Nishigaki S, Sato T, Wakiyama H, et al. Clinical results of trabectome surgery for open-angle glaucoma. Clinical Ophthalmology. 2015;9:1889-94. 14. Bussel II, Kaplowitz K, Schuman JS, Loewen NA. Trabectome Study Group. Outcomes of ab interno trabeculectomy with the trabectome by degree of angle opening. Br J Ophthalmol. 2015;99(7):914-9. 15. Resende AF, Patel NS, Waisbourd M, Katz LJ. iStent trabecular microbypass stent: An Update. J Ophthalmol. 2016;2016:2731856. 16. Neuhann TH. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: Long-term results. J Cataract Refract Surg. 2015 Dec;41(12):2664-71. 17. Malvankar-Mehta MS, Iordanous Y, Chen YN, et al. iStent with phacoemulsification versus phacoemulsification alone for patients with glaucoma and cataract: A meta-analysis. PLoS ONE. 2015;10(7):e0131770. 18. Vold S, Ahmed II, Craven ER, et al; CyPass Study Group.Two-year COMPASS trial results: Supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016 Aug 6. pii: S0161-6420(16)30500-0. [Epub ahead of print]. 19. Roberts SJ, Mulvahill M, SooHoo JR, et al. Efficacy of combined cataract extraction and endoscopic cyclophotocoagulation for the reduction of intraocular pressure and medication burden. Inter J Ophthalmol. 2016;9(5):693-8. 20. Francis BA, Berke SJ, Dustin L, et al. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg. 2014 Aug;40(8):1313–1321. 21. Seigal, Boling WS, Faridi OS, et al. Combined endoscopic cyclophotocoagulation and phacoemulsification versus phacoemulsification alone in the treatment of mild to moderate glaucoma. Clin Exper Ophthalmol. 2015 Aug;43(6):531-9. 22. Heijl A, Leske MC, Bengtsson B, et al. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002 Oct;120(10):1268-79. 23. De Keyser M, De Belder M, De Belder S, De Groot V. Where does selective laser trabeculoplasty stand now? A review. Eye and Vision. 2016;3:10. doi:10.1186/s40662-016-0041-y. 24. Song J. Complications of selective laser trabeculoplasty: a review. Clin Ophthalmol. 2016;10:137-43. 25. Jinapriya D, D’Souza M, Hollands H, et al. Anti-inflammatory therapy after selective laser trabeculoplasty: A randomized, double-masked, placebo-controlled clinical trial. Ophthalmology. 2014 Dec; 121(12):2356–61. 26. Krupin T, Stone RA, Cohen BH, et al: Acute intraocular pressure response to argon laser iridotomy. Ophthalmology. 1985;92:922-6. |

