
History
A 38-year-old black male reported to the office with a chief complaint of blurred vision, greater in the right eye than the left, of a year’s duration. His systemic history was non-contributory. His ocular history was remarkable for a retinal detachment in the left eye, secondary to blunt trauma sustained during a basketball game more than five years earlier with resultant traumatic open angle glaucoma in the left eye, medicated status post seton implantation eye, peripheral visual field constriction in the right eye with confrontational visual fields and a superior nasal field defect in the left eye. The biomicroscopic examination of the anterior segment of the right eye was normal.
The pertinent anterior segment findings of the left eye are demonstrated in the photograph (Figure 1). Goldmann applanation tonometry measured 11mm Hg OD and 20mm Hg OS. A dilated fundus examination of the right eye is demonstrated in a photograph (Figure 2). Undilated 90D fundus examination of the left eye demonstrated cup-disc ratios of 0.5/0.5 with diffuse pallor.
Diagnostic Data
His best corrected entering visual acuities were 20/400 OD and 20/40 OS at distance and near with no improvement upon pinhole or refraction. His external examination revealed a trace afferent pupillary defect in the right eye, peripheral visual field constriction in the right eye with confrontational visual fields and a superior nasal field defect in the left eye. The biomicroscopic examination of the anterior segment of the right eye was normal.
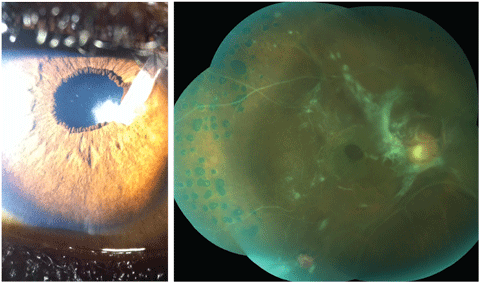 |
| Fig. 1 (left). This patient has had a number of ocular pathologies in the past, but can you tell what’s causing his blurred vision from this photograph? Fig. 2 (right). What can be learned from this fundus image of the patient’s left eye? Click image to enlarge. |
The pertinent anterior segment findings of the left eye are demonstrated in the photograph (Figure 1). Goldmann applanation tonometry measured 11mm Hg OD and 20mm Hg OS. A dilated fundus examination of the right eye is demonstrated in a photograph (Figure 2). Undilated 90D fundus examination of the left eye demonstrated cup-disc ratios of 0.5/0.5 with diffuse pallor.
Your Diagnosis
Does this case require any additional tests? What does this patient’s history and clinical findings tell you about her likely diagnosis?
Answers
Additional studies included photodocumentation of both the anterior and posterior segments, gonioscopy of both angles to rule out neovascularization, visual fields OS and prompt referral to retinology to rule out treatment for the right eye.
The diagnosis in this issue is cataract formation secondary to lenticular-seton contact, OS and evidence of old proliferative vitreoretinopathy secondary to previous traumatic/tractional retinal detachment.
Three forms of retinal detachment are recognized.1-9 They are: rhegmatogenous retinal detachment (RRD-resulting from fluid movement under a retinal break), exudative or serous retinal detachment (ERD-resulting from fluid accumulation under the sensory retina without a retinal break) and tractional retinal detachment (TRD-separation of the neurosensory retina from the retinal pigment epithelium resulting from the pull of proliferative and fibrovascular vitreal strands).1-11 Tractional retinal tears and detachments have no specific racial, gender or laterality predilection; rather they are a phenomeneon produced by complications of other pathologies which induce the intra/pre retinal fibrovascular proliferation.1-19 These diseases include but are not limited to proliferative diabetic retinopathy, ischemic venous occlusion, in rare instances retinal artery occlusion, ocular ischemic syndrome, proliferative sickle cell retinopathy, Eales’ disease, Coats’ disease and others treated with vascular endothelial growth factor (VEGF) inhibitors, pathological myopia, radiation retinopathy, retinopathy of prematurity, intermediate uveitis (pars planitis), proliferative vitreoretinopathy following rhegmatogenous retinal detachment repair, vitreomacular traction syndrome and epiretinal membrane.1-23 TRD has also been documented as a consequence of cryotherapy.24-26
Patients with TRD often report a sudden onset of either a single or multiple floating spots, along with flashing lights (photopsiae).27-31 Unlike entoptic phenomena which demonstrate exacerbations and remissions or the scintillating scotoma produced in vasospastic events, the visual symptoms remain stable in the patient's visual field.27-31 Pain is not a feature of any retinal detachment as the tissue has no pain receptors. There may be precipitating ocular or head trauma. If there has been a vitreous hemorrhage, there will be multiple large floaters or opacities which may take the form of “cob webs”.27-36 There may be a severe loss of vision if dense vitreous hemorrhage interrupts the visual axis or if the TRD involves the macula. It is also possible the patient is asymptomatic and unaware the condition has occurred.32
All retinal detachments involve a dissection of the neurosensory retina from its underlying retinal pigment epithelium (RPE) layer by subretinal fluid (SRF).6-9 The principle involved in TRD, weather it is secondary to proliferative vitreoretinopathy (PVR), trauma, vitreomacular traction syndrome, posterior vitreous detachment or retinal infiltration of choroidal neovascularization is that forces exerted by the vitreous or neovascularization at the site of their attachment to the retina overcome its tensile strength and separate it from the underlying RPE.6,12,13,14,20,34,36 Three main etiologies produce TRD, proliferative vitreoretinopathy, posterior vitreous detachment and vitreomacular traction syndrome (epiretinal membrane).27,42-71 Tractional retinal detachments do not have to occur with or produce a retinal break. When they do, this is termed a combined tractional/rhegmatogenous retinal detachment.
Proliferative vireoretinopathy has several associated models of pathogenesis.12,22,37-44 Each acknowledges the role of vitreous inflammatory cells, growth factors and cytokines as angiogenesis provoking agents.12,22,37-44 This process has the potential to be upregulated whenever ischemic retinal dynamics are present. This includes all retinal diseases capable of driving the process of neovascularization such as diabetic retinopathy, venous occlusion, artery occlusion, sickle cell retinopathy and others.1-22,27,37,51-63
Neovascular membranes attach to hyaloid face of the vitreous creating proliferative vascular networks. Vitreal shrinkage and strong anterior tractional forces result.37-40 RPE cell proliferation and migration are believed to play a role in the pathogenesis.38 Data suggests that the vitreous contributes modulators that stimulate RPE cells along with macrophages, fibroblasts and glial cells to interact with constituents of the extracellular matrix such as fibronectin, vitronectin and factor XIII.39,40 These mechanisms induce the formation of membranes that capture the sensory retina and forcibly separate it from the underlying RPE.37-43 Unlike rhegmatogenous or exudative retinal detachments which tend to occur acutely, TRD often develop slowly.40 At the molecular level, vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGF-B) seem to be the main drivers of angiogenesis and membrane contraction.41-44 VEGF upregulates the profibrotic connective tissue growth factor (CTGF) in newly formed neovascular membranes.42,43 Increasing levels of CTGF inactivate VEGF. When the balance tips beyond the threshold ratio, the neovascular membranes become more fibrotic and less vascular.43 Recently investigators have postulated that an imbalance between matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinases (TIMP proteins) may promote neovascularization via protein kinase-C (PKC) activation in retinal Müller cells.12
Finally, intravitreal anti-VEGF agents have gained popularity for the treatment of recalcitrant macular edema and intraocular neovascularization in all diseases that produce it.18,36,40,46 In addition, they have been used as presurgical adjuvants in diabetic vitrectomies and ROP. 45,46 Despite the advantages of such treatment when the agents produce rapid involution of the fibrovascular proliferation they have the potential to induce tractional retinal detachment.45,46
The processes of posterior vitreous detachment, epiretinal membrane formation and vireomacular traction syndrome are also commonly associated with TRD.53-60 The vitreous gel is formed by a dilute meshwork of collagen fibrils. These provide a scaffold-like structure formed by hyaluronic acid.28,47,48 Firm attachments of the vitreous to the retina occur via a “molecular glue” including laminin, fibronectin and sulfated proteoglycans in areas where the internal limiting membrane (ILM) is the thinnest such as the vitreous base, the margins of the optic disc, the back of the crystalline lens (hyloidocapsular ligament of Wieger), the diameter fovea, along large retinal vessels and sites of abnormal vitreoretinal adhesion such as lattice margins.27,49-51 The process of PVD begins with synchysis (liquification) of the vitreous and weakening of the vitreoretinal adhesions.27,52-55 As the vitreoretinal adhesions dissolve, discontinuities form within the posterior hyaloid (either via fissure evolution or via a microbreak in the thin cortical vitreous layer).56 This allows synchytic vitreous to enter the subhyaloid space dissecting the posterior hyaloid from the ILM.30,50,51,58,59 An anomalous PVD (APVD) results when synchysis occurs without sufficient detachment from the ILM.52 This results in tractional effects at the interface (epiretinal membrane-ERM).51 The physics of anomalous PVD has the potential to generate forces which split the posterior vitreous cortex causing vitreoschesis.52 When this phenomenon occurs in the periphery, tractional forces increase the risk of TRD, retinal tears and combined detachments, especially at the margins of anatomically thin retina (lattice degeneration).51,52,59 When these forces project adjacent to the macula, it has the potential to induce wrinkling of the neurosensory retina referred to as macular pucker.52,59,60 The pathology is also known as cellophane maculopathy, epiretinal membrane, vitreoretinal interface maculopathy and vitreo-macular traction syndrome (VMTS).52,59,60 Over time, as traction increases in either force or size, it can create a dehiscence (a break) that initiates the formation of macular hole.52,59-61 Microscopic examinations of epiretinal membranes have revealed that retinal pigment epithelial (RPE) cells, glial cells, fibrocytes, macrophages, laminocytes and collagen fibrils are all players in their formation.59,61-64 Hyperconvolution and duplication of the ILM which occurs during the development of epiretinal membrane are distinctive features which permit it to exert tractional forces on the retina, increasing the risk of TRD or combination detachment.63,64
The management of a TRD depends upon weather there is an associated retinal break (combined tractional/rhegmatogenous retinal detachment).69-71 In cases where there is only tractional retinal separation without a retinal break, a three-port pars plana vitrectomy (sclerotomies for infusion, lighting and instrumentation) can be completed to release the tangential forces with retinal support promoted by the injection of a tapenade agent (expansile perfluorocarbon gas [perfluorohexane,perfluoropropane], air or silicone oil) to flatten the retina.1,4,51,61,63,65-68 In all surgical cases, topical cycloplegic, antibiotic and steroidal drops are prescribed post operatively.
In cases of epiretinal membrane, traditional treatment for symptomatic patients with reduced acuity includes 3-port pars plana vitrectomy and membrane peeling with or without adjunct injectable steroid.66,67,72 In some instances surgeons use indocyanine green dye or other dyes (brilliant blue G-BBG) to stain the ILM, clarifying the boundaries of the ERM.66,73 Ironically, intravitreal steroids also increase the risk of TRD.
Vitreomacular traction syndrome is treated similarly, using 3-port pars plana vitrectomy to release the debilitating adhesive forces.67 Unique to this condition are additional preretinal layers which may represent a thickened posterior hyaloid interface.67,74 If an epiretinal membrane is present it too may require peeling.67,74-76 A novel pharmaceutical approach has been developed using an intravitreal injection of the recombinant protease agent Jetrea (Ocriplasmin, ThromboGenics).75,76 The agent has targeted activity against fibronectin and laminin which are the primary components of the vitreoretinal interface.75,76 The agent has shown promise against the placebo in testing for releasing vitreous traction (25% published and anecdotally) and closing macular holes (40% published and anecdotally).76 Today, parsplana vitrectomy remains the standard-of-care for closing macular holes.76,77
When combination detachments occur (secondary to vascular proliferative disease, at sites of lattice degeneration or macular hole formation) surgical procedures are designed with four goals: close each retinal break, remove vireoretinal traction sources, reattach the retina and eliminate counterproductive vitreous fluid dynamics.1,4,51,61,63,65-69,70 Here, the fibrovascular proliferation or proliferative vireoretinopathy (PVR-non-vascular membranes) are repaired using the techniques of segmentation, delamination and en bloc dissection in combination with the use of perfluorocarbon liquids to flatten the retinal tears, visualize membrane traction and force the subretinal fluid (SRF) out from underneath the neurosensory retina.69 For instances where additional SRF removal is required, retinotomies may be necessary.1 The final step in these cases is creating a thickened RPE-retinal interface 360 degrees around the each retinal break so that no additional fluid may permeate underneath the photoreceptors. This is typically done with binocular indirect laser application and is termed endophotocoagulation.71 Adding tamponade to keep the retina flat during healing is typical in macular repair. In other cases its use can be determined by the extent of the injury.71
|

