 |
History
A 66-year-old woman came to the office with a chief complaint of dry, scratchy eyes which she had been experiencing for the previous six months. She explained that the problem had been worsening despite her general practitioner treating her with artificial tear drops.
She had no known previous ocular disease and reported a systemic history of hypertension for which she was properly medicated with lisinopril. She reported no allergies.
Diagnostic Data
Her best-corrected visual acuities were 20/30 OD and 20/30 OS at distance and 20/40 at near, both eyes. External examination was normal and there was no evidence of afferent pupillary defect. Refraction revealed symmetrical, spherical myopia with a changes in the carrier and add that yielded 20/25 acuity at distance and near. The pertinent anterior segment findings are demonstrated in the photograph. Intraocular pressures were measured with Goldman applanation at 19mm Hg, OU. The dilated fundus examination revealed normal posterior poles, without evidence of choroidal folds, hypertensive retinopathy or peripheral pathology.
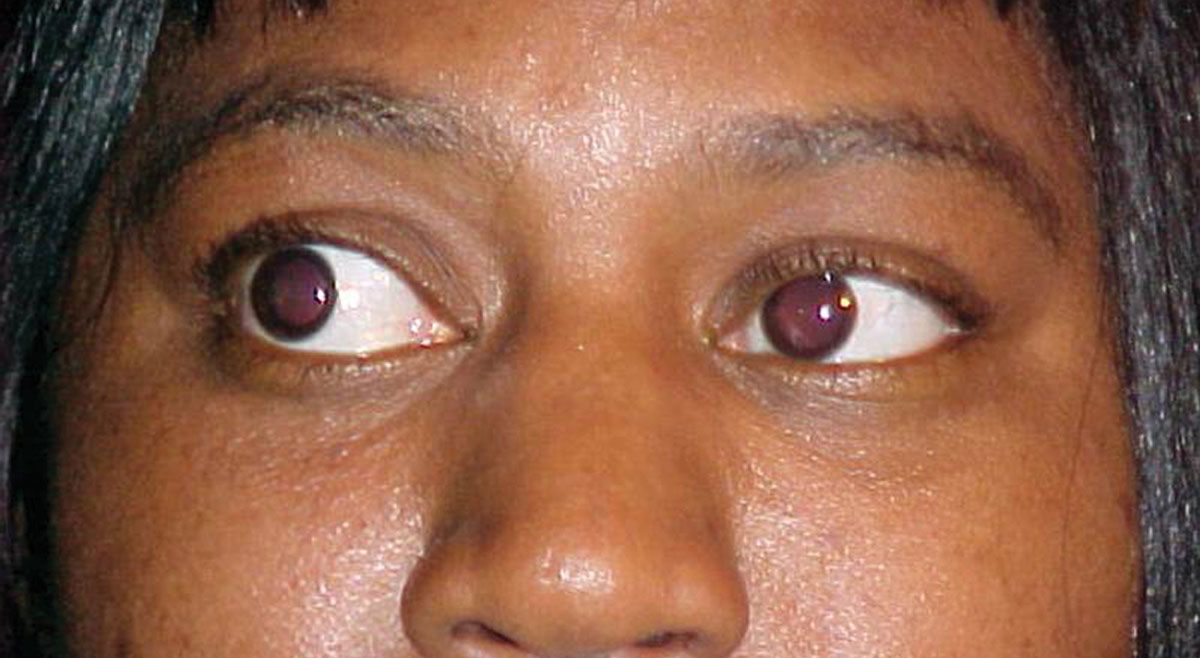 |
| What can this patient’s anterior segment presentation tell you about the likely cause of her dry and scratchy eyes? Click image to enlarge. |
Diagnosis
Additional tests and procedures included Hertel exophtalmometry (24,25 with a base of 102), sodium fluorescein dye testing uncovering mild inferior punctuate keratopathy, lacrimal lake observation and Schirmer tear testing to assay the quantity and quality of the tear layer and retropulsion of the globes to insure free movement and rule out a posterior mass effect. The patient was treated with increased viscosity artificial tear preparations known to have increased contact time as well as artificial tear drops of superior quality throughout the day and as needed. Punctal plugs and Restasis (cyclosporine, Allergan) were discussed as potential future interventions. Correspondence was sent to the patient’s general physician requesting thyroid testing (T3, T4, TSH, thyroid perioxidase antibody testing, thyroglobin testing and CT scans of the orbits to rule out tendon sparing extraocular muscle involvement).
The topical treatments greatly improved the patient’s comfort and sight. The other interventions will be considered in the future if symptoms return and cannot be mitigated with conservative measures.
The systemic test schedule uncovered mild thyroid eye disease, resulting in a referral to an endocrinologist. The proper systemic management was initiated by the endocrinology team.
The diagnosis in this months issue is thyroid eye disease.
Graves' disease, Graves orbitopathy, dysthyroid orbitopathy, thyroid eye disease and thyroid ophthalmopathy (TO) are all synonymous terms connoting an autoimmune disorder characterized by multiple systemic manifestations.1,2
Discussion
Thyroid ophthalmopathy demonstrates some form of clinical relevance in approximately 50% of patients with systemic thyroid dydfunction.3 It has the potential to affect 3% to 5% of patients with advanced sequellae.3 The fifth and seventh decades of life represent age peaks of incidence with a slight preponderance for women over men.3 The natural history of the disease remains poorly defined.1-4 In fact, the ocular aspects of the disease may remit or improve spontaneously.3 The ocular conditions characteristic of the disease may exist in the absence of clinical or biochemical evidence of thyroid dysfunction.2,3 When the systemic and ocular condition exist together they may follow completely different clinical courses.2-6
The hallmark sign of TO is bilateral, non-pulsatile proptosis secondary to tendon sparring extraocular muscle enlargement.1-4 Other important findings include impaired ocular motility, firm resistance to globe retropulsion, eye lid retraction, poor upper eye lid tracking upon down gaze (Von Graefe’s sign), variable diplopia, variably impaired visual acuity, sight loss secondary to corneal exposure-related keratopathy or compressive optic neuropathy with visual field defects.7,8
The dichotomy of thyroid disease, in general, is its often unpredictable nature. As an example, dermopathy has been observed in many cases without any clinical ophthalmopathy.5
The etiology of the disorder is multifactorial and secondary to newly discovered heritable abnormalities which interfere with immune regulation.1 Not all of the mechanisms responsible for the systemic and/or ocular manifestations are understood.2 Research regarding the clinical manifestations of thyroid ophthalmopathy traditionally point to a combination of increased orbital fat and extraocular muscle volume within the orbital space.1,2,9-12
TO is a slowly progressive disease where a malfunctioning immune system activates thyrotropin receptor-specific T and B cells.11 This activation produces autoantibodies.10 Orbital fibroblasts residing within the orbital tissues succumb to autoimmune attack when these antibodies bind to both their thyrotropin receptors (TSHr) and insulin-like growth factor-1 (IGF-1) receptors.9-12 Orbital fibroblasts act as sentinel cells, initiating lymphocyte recruitment and tissue remodeling.6 Evidence suggests that when the autoantibodies bind to the thyrotropin receptor cites they stimulate a subset of these cells to undergo adipogenesis increasing orbital adipose tissue volume.9,10 Autoantibodies which bind to the insulin-like growth factor-1 receptors appear to impact pathogenesis through recruitment and activation of additional T-cells and by upregulating the production of hyaluronan (hyaluronic acid), a cytokine which plays a key role in the development of inflammation and increased orbital tissue swelling.9,10 Simultaneously, the process stimulates an overproduction of thyroid hormones.9-12 Activated T-cells further infiltrate orbital tissues, further kindling the cascade of inflammation.9,10 Finally, although originally thought to represent a separate pathway for inflammation, antibodies which develop against the extraocular muscles are now considered to be a secondary consequence of global extraocular muscle inflammation.9
Thyroglobulin (Tg) may also be involved in the pathogenesis of TO.13,14 Following its release from the cytokine stimulated thyroid, Tg may have the ability to elicit autoimmune aggression in orbital tissues and extraocular muscles by becoming complexed with glycosaminoglycans, infiltrating the tissues and then serving as a binding cite for the other previously mentioned cytokines.13,14 This mechanism was traditionally viewed as the main pathway of pathogenesis, however, recent research has relegated it as a secondary player.13
Interestingly, environmental triggers, such as smoking, have been shown to have the ability to induce or hasten ophthalmopathy.3,15,16 Since smoking is a modifiable risk factor, it must be included in the historical questioning and then approached in management.15-17
The management of patients with suspected TO have a duel consideration. Since the hallmark sign of TO is a bilateral, symmetrical, proptotic appearance, laboratory testing and imaging is indicated to understand the potential etiology in suspicious appearing patients.18 However, since ophthalmopathy can develop in the setting of an under active, hyper active or normally functioning thyroid gland, contingencies for treating glandular malfunction and eye signs and symptoms must be developed separately.1-4,7,17-25
Laboratory testing should include assays for the levels of triidothyronine (T3), thyroxine (T4), thyroid stimulating hormone (TSH), thyroid peroxidase antibodies, thyroglobin antibodies, thyrotropin receptor antibodies and thyroid stimulating immunoglobulins.2,3 While ultrasonography can be used to assess the condition of the extraocular muscles, computed tomography (CT) is a generally considered the accepted alternative for prechiasmal examinations. Magnetic resonance imaging (MRI) is the study of choice for those who require a detailed view of the soft tissues of the entire orbit.18 Monitoring of the condition should be accomplished with either a Ludde or Hertel exophthalmometer and or photographs.
Treatment
Any signs and symptoms associated with ocular surface compromise can be belayed with efficient hydration.25,26 Tear replacement/augmentation solutions and ointments can be prescribed to remedy most circumstances.25,26 Punctual occlusion can be used to retard drainage as well. Patients, with increased symptoms upon waking, may not be sleeping with their eye lids completely closed. In these instances patients can be counseled to use a blind fold to create an enclosed, moist environment and prevent exposure-based corneal abrasion. In the most severe cases, moisture chambers can be created for the waking hours by manufacturing moisture chambers by affixing face-conforming plastic barriers to the temples of the patient’s spectacles. Affixed Fresnel or ground-in prisms can be used to improve fusion in patients with limited motility.26 In rare instances congestion of the orbit can lead to raised intraocular pressure. Here, topical hypotensives may be appropriate.
Clincal trials with oral montelukast and oral cetirizine, antihistamine/antileukotriene preparations, respectively, traditionally used in the treatment of nasal itching, rhinorrhea and congestion, have demonstrated some effectiveness for patients with mild to moderate thyroid eye disease and orbital congestion.19 A six-week course of oral cetirizine (10mg every QAM) and oral montelukast (10mg QPM) in a series of patients with significant ophthalmopathy yielded subjective improvement in 50% of subjects compared with controls for tearing, dryness and itching with less effect on diplopia and proptosis.19
Oral and intravenous steroids have been demonstrated as successful therapies for cases of moderate to severe ophthalmopathy as general immunosuppressants.20,21,26 Work has shown that blocking the CD-20 receptor on B lymphocytes has significantly affected the clinical course of TO, resulting in rapid reduction of inflammation and proptosis.20
Experimental use of infliximab (anti-tumor necrosis factor alpha) has also been investigated in TO patients secondary to its ability to decrease inflammation.22 While the option is a reasonable alternative to surgical approaches, the data on this new strategy, at present, is limited with respect to thyroid eye disease and orbital inflammatory conditions.22
In cases where TO is associated with a thyroid gland that is overactive, various antithyroid medications can be employed with or without the possibility of radioiodine treatment.25,26
The combination of corticosteroid treatment and external beam radiation to the thyroid gland is another effective modality where multiple mechanisms can be placated.21
Orbital radiation is a controversial local treatment modality for patients with severe thyroid ophthalmopathy.24,27 The literature demonstrates a lack of consensus and standardization with variable quality of published reports.24,27 At best, the therapy offers an option for those patients with extraocular motility impairment, however, even that indication has received mixed conclusions with clinical trial evidence indicating that proptosis, eyelid retraction and soft tissue changes may not improve with radiation treatment.5,24,27
Orbital decompression by transpalpebral fat removal is a proven, reliable, effective and safe method of relieving compressive complications of TO. The procedure has developed a track record for lasting results, improvement in visual function and development of patient well-being with a high-benefit-to-low-risk ratio.7
|

