 |
A 74-year woman presented to the office with a chief complaint of “blurred vision for months.” She said the issue had gradually become worse over time. Her ocular history was positive for cataract removal with intraocular lens implantation three years prior. She did not report any pain. She denied trauma, systemic disease and allergies of any kind.
Diagnostic Data
Her best-corrected entering visual acuities were 20/30 OD and 20/30 OS. Her external examination was unremarkable with no evidence of afferent pupillary defect. The biomicroscopic examination was normal with no posterior capsular opacification and a centered lens. Her Goldmann applanation tonometry measured 17mm Hg OU.
Additional studies included color photodocumentation, laser interferometry to assess best capable function, optical coherence tomography (OCT) to understand retinal status, OCT angiography to rule out choroidal neovascularization and fluorescein angiography to rule out the presence of choroidal neovascularization and/or retinal pigment epithelial cell damage or leakage.
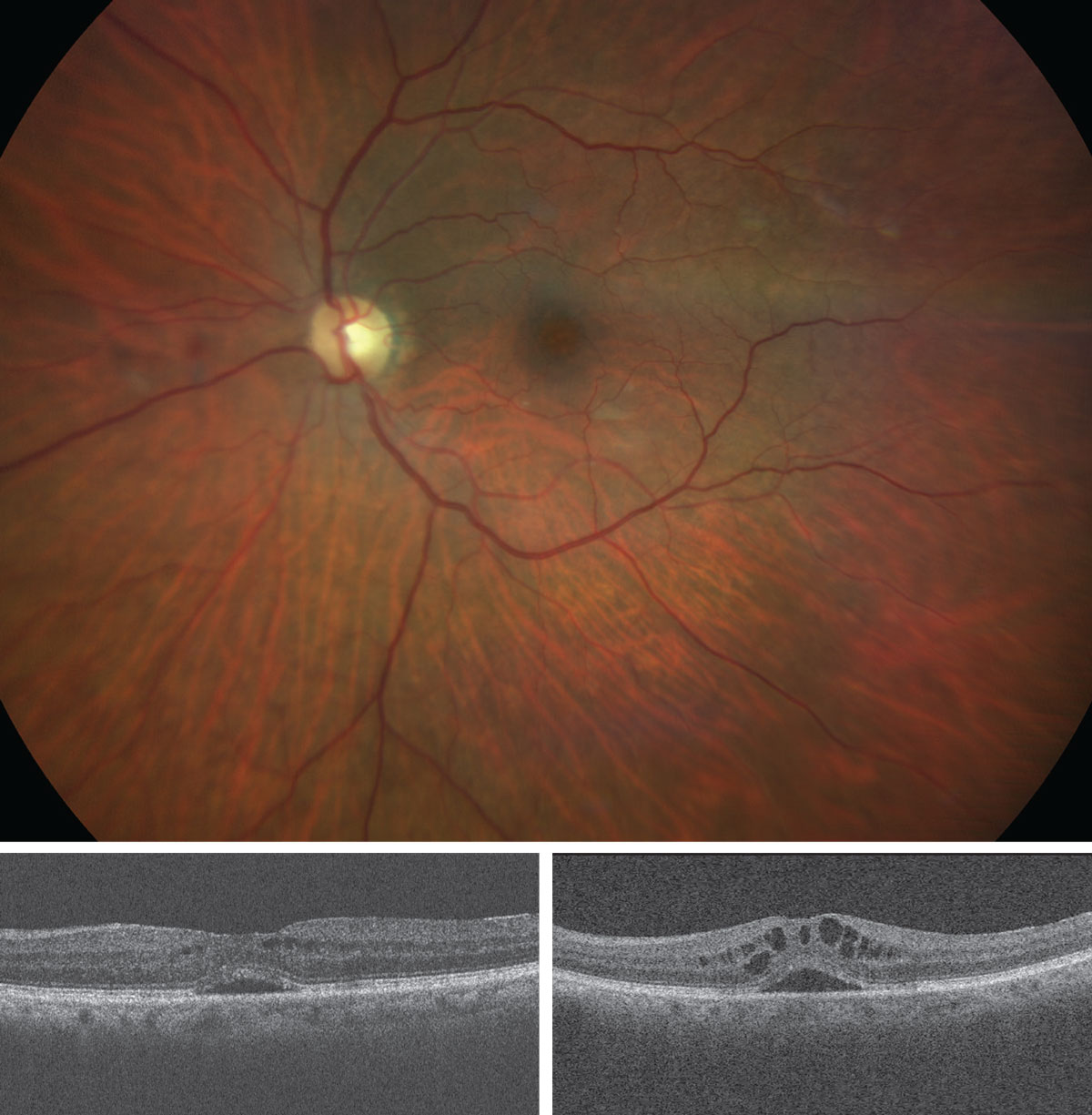 |
| What do these findings suggest about the patient? How would you approach management? Click image to enlarge. |
The pertinent posterior segment findings are demonstrated above.
Making the Call
The condition here is “late” cystic macular edema (CME) secondary to cataract surgery, OS. Cystoid or cystic macular edema (CME) is not a true diagnosis but rather a finding occurring from numerous causes.
CME is named for its histopathologic and now OCT appearance: small, round and oval intraretinal spaces (cysts) filled with fluid. Many retinal conditions (e.g., diabetes, artery occlusion, vein occlusion, traumatic retinopathy) have the capability of producing intraretinal vascular incompetence leading to small fluid laden cavities. Traditionally, the issue is associated with patients that have undergone cataract surgery (or some other invasive ocular surgery).1-18
The term Irvine-Gass syndrome was coined to connote CME following cataract surgery.1-6 The term was specifically meant to identify the “petaloid” (like the petals of a flower) fluorescein angiography results seen after fluid had filled into intraretinal cystic spaces surrounding the macula post complicated intracapsular cataract extraction which experienced some vitreous loss.2-5
Today, the term CME connotes any cystic macular edema whenever or it is discovered; when discovered outside the macula, it is termed cystic retinal edema.6,7
How it Occurs
Causative factors beyond cataract surgery can include ocular eyedrop preservatives, topical prostaglandin analogs, topical beta-blockers, retinal vein occlusion, diabetes, central serous chorioretinopathy, anterior or posterior uveitis, pars planitis, retinitis pigmentosa, radiation retinopathy, posterior vitreous detachment, epiretinal membrane formation, macular retinal telangiectasia, post YAG laser procedure and blunt trauma, to name a few.2-18 Given the broad base of potential causative pathologies—with the exception of cataract surgery, where some hard predictive data exists—the epidemiology for the formation CME rests with the particulars of the inciting disease.2-18 After cataract surgery, the second most common cause of CME is diabetes.2
Historically speaking, pseudophakic cystoid macular edema (PCME) was first described in 1953 by A. Ray Irvine, Jr., who observed that some patients had unexplained visual loss following intracapsular cataract extraction.4,5 The underlying cause of the visual loss was later identified by Gass and Norton.2,4,5 They added to the work of Irvine, documenting a phenomenon exhibiting what they named the “perifoveal petalloid pattern” of staining along with late leakage from the optic nerve upon intravenous fluorescein angiography (IVFA).4,5 The condition came to be known by their names.2
Today, the incidence of PCME has decreased significantly for several reasons: the transition from intracapsular cataract surgery to small incision, clear corneal extracapsular cataract surgery using phacoemulsification; the deployment of small incision foldable lenses; the improvement of technology enabling less intraoperative energy use and faster surgical times with better intraoperative cushioning agents; use of preoperative anti-infective/anti-inflammatory therapeutics and the development and deployment of better topical anti-inflammatory agents.
While the modern incidence of PCME-related symptoms (defined as vision loss 20/40 or worse) is low (approximately 0.1% to 2.35% of all cases), an estimated 20% to 30% of patients undergoing phacoemulsification would demonstrate some form of mild PCME if IVFA were completed.4 The rate has been estimated as high as 41% using OCT.3 Fortunately, the patients who would have PCME only detected upon IVFA or OCT imaging often have no visual disturbances and require no intervention.4
Late-onset CME has been noted to occur in only a small percentage of cases.19 The longest postoperative onset of CME, related to a distant cataract procedure documented in the literature, is 17 years.19 Continue this with additional info from your research.
Clinical Impact
The predominant symptom caused by CME of any etiology is visual distortion (metamorphopsia) and acuity reduction.2-18 Visual acuity may be minimally reduced or can decrease to 20/400.3-18 Depending upon the underlying cause of CME, the patient may also manifest signs and symptoms that are related to the inciting disease.2-18
The ophthalmoscopic appearance of perifoveal retinal thickening is difficult to observe. The normal appearance of retinal tissue should be transparent and flat. Edematous retinal tissue can be stereoscopically appreciated using indirect biomicroscopic technique, as being raised, having depth, with an opalescence contributing to both the tissue’s cloudiness and an inability to discern underlying choroidal detail.2
In severe cases, intraretinal cysts and the gathering of luteal pigment can create a radiating or oval yellow nodule in the region of the macula.2 With indirect lighting, a honeycombed appearance may be appreciable corresponding to the delineation of the individual fluid filled cysts.2
The compromised foveo-macular architecture often causes the elimination of the foveal light reflex. The true “petaloid” appearance of CME is best appreciated with fluorescein angiography.2-4 OCT testing is preferred when possible as it permits noninvasive observation of the cystic, fluid-filled spaces.6
CME is not a specific disease, but rather a clinical feature that occurs as complication of a number of retinal conditions. Intracellular fluid and Müller cell swelling produce the condition’s distinctive hexagonal appearance.2 When the fluid remains intracellular, the effects of the disruption remain reversible.2 Once the cellular membranes rupture, giving rise to extracellular leakage, the damage is both irreversible and more significant.2
Leaking perifoveal capillaries, subject to the pathophysiology of the underlying cause, go on to create the formation of intraretinal polycystic fluid-filled spaces, which disrupt light from reaching the photoreceptors. This also delays efficient dialogue with the visual pathway.2-4 Exudative or transudative fluid collects in the loosely arranged outer plexiform layer of Henle, where the axons of the photoreceptors synapse with the dendrites of the horizontal, bipolar and amacrine cells in the macular clivi. The fibers in the macula’s Henle’s layer are arranged so that they are maneuvered to the sides, allowing maximum light transmission directly to the photorecptors. This is what creates the foveal clivus and the characteristic parabolic shape. This anatomy, along with the sequential filling of created cysts, is what creates the “petaloid” appearance seen during fluorescein angiography.3,4
Inflammatory mediators disrupt the blood-aqueous barrier (and blood-retinal barrier), leading to increased vascular permeability.2-4 Any disease process that can breakdown these barriers can induce CME.2-21 Surgical manipulation may lead to the excessive release of arachidonic acid from cell membranes with production of either leukotrienes via the lipooxygenase pathway or prostaglandins via the cyclooxygenase pathway.2-4 These inflammatory biomarkers can result in increased retinal vessel permeability and the development of CME.2-21 Light toxicity from the operating microscope may contribute to free radical release with subsequent prostaglandin synthesis.2-5 Prostaglandins contribute to tissue inflammation increasing vasodilation and vasopermeability.2
Any contraction of the posterior hyaloid membrane as a result of epiretinal membrane formation secondary to surgical procedures, inflammation from disease processes, anomalous posterior vitreous detachment, or vitreomacular adhesions may lead to traction onto the perifoveal retinal capillaries and the vasogenic and cytotoxic factors that produce CME.2,21
In cases of CME occurring from any form of uveitis, it can logically be assumed that the inflammatory process initiated by released prostaglandins contributed to perifoveal capillary dilation with increased permeability and fluid exudation.2,7,20 The same reasoning can be extended to CME occurring secondary to prostaglandin analog use in the management of glaucoma. This complication is more prevalent in patients that have undergone incisional surgery and who have an opened posterior capsule, which, theoretically, allows easier access of cytokines into the eye.2,9,10
Chronic CME can permanently alter macular architecture.24 This transformation is accompanied by a substantial reduction in macular thickness known as a lamellar macular hole (LH).2,2124 LH typically do not lead to changes in visual function.24 There have been cases of full-thickness holes resulting from CME treatments with injectable steroids such as triamcinolone.25
Intervention Options
CME is best managed by the cataract surgeon-of-record or the confirming retina subspecialist. However, it can be monitored and measured by optometrists. When CME is caused by conditions such as diabetes, retinal vein occlusion, retinitis pigmentosa or uveitis, the treatment is dictated by standards of care for the causative condition.2-34 Cases of CME arising from diabetic retinopathy or retinal vein occlusion would warrant consideration of focal laser photocoagulation of the leaking perifoveal capillaries, alone or in combination with injections of anti-VEGF agents. Intravitreal steroid injections (fluocinolone acetonide, triamcinolone, anecortave acetate) or an intravitreal steroid implant (dexamethasone) is plausible as well for cases that do not resolve with the other modalities.
Therapy is determined using the guidelines of both new and classic studies.26-30 In inflammatory diseases such as iritis, uveitis, pars planitis (intermediate uveitis), scleritis and retinitis, topical cycloplegics such as atropine 1% BID-TID, along with topical and oral nonsteroidal anti-inflammatory drugs, corticosteroids, immunosuppressants, laser photocoagulation and VEGFI can be used.31-33
The majority of cases of symptomatic Irvine-Gass syndrome resolve spontaneously without specific intervention within eight months.2-6,34 Fifty percent of cases resolve sooner.2-6 In rare instances, cases can remain angiographically or OCT detectable in excess of five years.2
Medications for CME include the oral nonsteroidal medicines ibuprofen and indomethacin and the corticosteroid prednisone. Topical nonsteroidal medications such as ketorolac, nevenac and bromfenac have also been used successfully. Topical corticosteroid drops such as prednisolone acetate, loteprednol etabonate and difluprednate can be added for unresponsive or severe cases.2-5,34,35
Common dosing ranges from QID to Q2h. Often, a loading dose of Q2h is initiated and then rapidly dropped to QID after several days. Duration of therapy may be several days to months, depending upon the severity of the CME.2-18,34,35
Oral carbonic anhydrase inhibitors like acetazolamide and methazolamide have been documented as helpful in recalcitrant cases of CME.36 These agents increase active transport by the retinal pigment epithelium to facilitate fluid movement from the retina through the choroid.36 They work best in cases caused by diffuse retinal pigment epithelial failure (retinal dystrophies).36 The use of these agents is limited to the patient’s ability to tolerate the medication and its linty of side effects.36 The community has concluded an eight-week trial is not unreasonable.36
The majority of cases of symptomatic CME following cataract surgery resolve spontaneously without intervention within eight months, and many cases resolve faster.2-6,34 In rare instances, CME can remain angiographically or tomographically detectable in excess of five years, though patients may not be visually disturbed.2
In cases where vitreous traction has induced or contributed to the formation of CME, surgical vitrectomy has demonstrated success.37 New endoscopic laser delivery systems have permitted surgeons the option of shaving the vitreous without having to complete removal.37 The effectiveness of vitreous surgery with internal limiting membrane (ILM) peeling stems from relief of posterior hyaloid membrane traction, removal of inflammatory cytokines, while increasing preretinal oxygen pressure.37 It is hypothesized that the ILM is the basement membrane of the Müller cells and may act as a diffusion barrier, decreasing transretinal fluid movement.37
New investigations seek to duplicate the results seen in vitrectomy using intravitreal pharmacologic agents.37 A carbamide derivative (Vitreosolve, Innovations in Sight) is currently being evaluated in Phase III randomized controlled trials in patients with nonproliferative diabetic retinopathy.37 Microplasmin (Jetrea, Thrombogenics), an intravitreally injected fragment of plasmin, is currently used to treat vitreomacular traction syndrome and is also being studied as a treatment for diabetic macular edema in a sham-controlled trial.37
Surgeons have found synergistic effects by mixing radial sheath optic neurotomy, pars plana vitrectomy with ILM peeling and postoperative intravitreal triamcinolone injection for the treatment of continuing retinal vein occlusion-induced CME.37
While vitrectomy is not free of risks and will induce premature cataractogenesis eventually requiring lensectomy, it is worth considering in uveitic and pediatric cases, as it improves the visual condition and allows systemic immunosuppressive therapies to be decreased.37
Upbeat Prognosis
CME remains a complication of cataract extraction even in uncomplicated cases. CME following a cataract procedure is more likely to occur in cases where the capsule has been ruptured or the vitreous incarcerated. When oral or topical steroids are used, intraocular pressure must be monitored. If the pressure rises, it must be treated with an ocular hypotensive that has a low risk for aggravating the condition. As such, avoid prostaglandin analogs.
Amsler grid home monitoring can be used to track the progress of recovery and insure condition stability. OCT testing can be used in office. Prostaglandin analogs should be used with caution in patients with a history of incisional ocular surgery, especially if there is also a broken posterior capsule. If left untreated, the fluid-filled cysts in CME may coalesce to form a macular cyst or lamellar hole.
Our patient was discovered to have significant macular pucker without frank vitreomacular adhesion or traction syndrome. That is as good reason as any to understand why this case developed. She was treated with topical anti-inflammatory eye drops and resolved well over time, not requiring surgical intervention. Her acuity remains at the 20/30 level.
Dr. Gurwood thanks Alice Lim, OD, and Nick Karbach, OD, for their contributions to this case.
Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is attending medical staff in the department of ophthalmology at Albert Einstein Medical Center, Philadelphia. He has no financial interests to disclose.
1. http://www.merriam-webster.com/dictionary/cyst.2. Fu A, Ahmed I, Al E. Cystoid macular edema. In: Yanoff M, Duker JS. Ophthalmology. Mosby-Elsevier, St. Louis, MO 2009:956-62.
3. Arshinoff SA. Same-day cataract surgery should be the standard of care for patients with bilateral visually significant cataract. Surv Ophthalmol. 2012;57(6):574-9.
4. Guo S, Patel S, Baumrind B, et al. Management of pseudophakic cystoid macular edema. Surv Ophthalmol. 2014;pii:S0039-6257(14)00178-7.
5. Gass JD, Norton EW. Cystoid macular edema and papilledema following cataract extraction. A fluorescein fundoscopic and angiographic study. Arch Ophthalmol. 1966;76(6):646–61.
6. Trichonas G, Kaiser PK. Optical coherence tomography imaging of macular oedema. Br J Ophthalmol. 2014;98 Suppl 2:ii24-9.
7. Fardeau C, Champion E, Massamba N, LeHoang P. Uveitic macular edema. J Fr Ophtalmol. 2015;38(1):74-81.
8. Sigler EJ. Microcysts in the inner nuclear layer, a nonspecific SD-OCT sign of cystoid macular edema. Invest Ophthalmol Vis Sci. 2014;55(5):3282-4.
9. Rosin LM, Bell NP. Preservative toxicity in glaucoma medication: clinical evaluation of benzalkonium chloride-free 0.5% timolol eye drops. Clin Ophthalmol. 2013;7(10):2131-5.
10. Matsuura K, Sasaki S, Uotani R. Successful treatment of prostaglandin-induced cystoid macular edema with subtenon triamcinolone. Clin Ophthalmol. 2012;6(12):2105-8.
11. Song SJ, Wong TY. Current concepts in diabetic retinopathy. Diabetes Metab J. 2014;38(6):416-25.
12. Arevalo JF. Diabetic macular edema: changing treatment paradigms. Curr Opin Ophthalmol. 2014;25(6):502-7.
13. Sarao V, Bertoli F, Veritti D, Lanzetta P. Pharmacotherapy for treatment of retinal vein occlusion. Expert Opin Pharmacother. 2014;15(16):2373-84.
14. Ahn SJ, Ryoo NK, Woo SJ. Ocular toxocariasis: clinical features, diagnosis, treatment, and prevention. Asia Pac Allergy. 2014;4(3):134-41.
15. Triantafylla M, Massa HF, Dardabounis D, et al. Ranibizumab for the treatment of degenerative ocular conditions. Clin Ophthalmol. 2014;24;(8):1187-98.
16. Frisina R, Pinackatt SJ, Sartore M, et al. Cystoid macular edema after pars plana vitrectomy for idiopathic epiretinal membrane. Graefes Arch Clin Exp Ophthalmol. 2015;253(1):47-56.
17. Kim JW, Choi KS. Quantitative analysis of macular contraction in idiopathic epiretinal membrane. BMC Ophthalmol. 2014;14(1):51.
18. Bagnis A, Saccà SC, Iester M, Traverso CE. Cystoid macular edema after cataract surgery in a patient with previous severe iritis following argon laser peripheral iridoplasty. Clin Ophthalmol. 2011;5(4):473-6.
19. Shah SU, Shields CL, Bianciotto CG, et al. Intravitreal bevacizumab at 4-month intervals for prevention of macular edema after plaque radiotherapy of uveal melanoma. Ophthalmology. 2014;121(1):269-75.
20. Yu S, Yannuzzi LA. Bilateral perifoveal macular ischemia in sarcoidosis. Retin Cases Brief Rep. 2014;8(3):212-4.
21. Pop M, Gheorghe A. Pathology of the vitreomacular interface. Oftalmologia. 2014;58(2):3-7.
22. Rezaei Kanavi M, Soheilian M.Histopathologic and electron microscopic features of internal limiting membranes in maculopathies of various etiologies. J Ophthalmic Vis Res.2014;9(2):215-22.
23. Pang CE, Spaide RF, Freund KB. Epiretinal proliferation seen in association with lamellar macular holes: a distinct clinical entity. Retina. 2014;34(8):1513-23.
24. Tsukada K, Tsujikawa A, Murakami T, et al. Lamellar macular hole formation in chronic cystoid macular edema associated with retinal vein occlusion. Jpn J Ophthalmol. 2011;55(5):506-13.
25. Lecleire-Collet A, Offret O, Gaucher D, et al. Full-thickness macular hole in a patient with diabetic cystoid macular oedema treated by intravitreal triamcinolone injections. Acta Ophthalmol Scand. 2007;85(7):795-8.
26. Vujosevic S, Martini F, Convento E, et al. Subthreshold laser therapy for diabetic macular edema: metabolic and safety issues. Curr Med Chem. 2013;20(26):3267-71.
27. Ford JA, Elders A, Shyangdan D,et al. The relative clinical effectiveness of ranibizumab and bevacizumab in diabetic macular oedema: an indirect comparison in a systematic review. BMJ. 2012;345(8):e5182.
28. Giuliari GP. Diabetic retinopathy: current and new treatment options. Curr Diabetes Rev.
2012;8(1):32-41.
29. Pielen A, Feltgen N, Isserstedt C, et al. Efficacy and safety of intravitreal therapy in macular edema due to branch and central retinal vein occlusion: a systematic review. PLoS One. 2013;8(10):e78538.
30. Lambiase A, Abdolrahimzadeh S, Recupero SM. An update on intravitreal implants in use for eye disorders. Drugs Today (Barc). 2014;50(3):239-49.
31. Zierhut M, Abu El-Asrar AM, Bodaghi B, Tugal-Tutkun I. Therapy of ocular Behçet disease. Ocul Immunol Inflamm. 2014;22(1):64-76.
32. Sigler EJ, Randolph JC, Calzada JI, Current management of Coats disease. Surv Ophthalmol. 2014;59(1):30-46.
33. Bodaghi B, Touitou V, Fardeau C,et al. Ocular sarcoidosis. Presse Med. 2012;41(6 Pt 2):e349-54.
34. Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol. 2012;23(1):26-32.
35. Kang-Mieler JJ, Osswald CR, Mieler WF. Advances in ocular drug delivery: emphasis on the posterior segment. Expert Opin Drug Deliv. 2014;11(10):1647-60.
36. Salvatore S, Fishman GA, Genead MA. Treatment of cystic macular lesions in hereditary retinal dystrophies. Surv Ophthalmol. 2013;58(6):560-84.
37. Golan S, Loewenstein A. Surgical treatment for macular edema. Semin Ophthalmol. 2014;29(4):242-56.

