 |
History
A 65-year-old black male presented with a chief complaint of blurry vision in his left eye. He had been experiencing this issue for two months. He denied any history of trauma or previous ocular illness. His medical history was remarkable for hypertension, for which he was medicated with lisinopril daily. He also denied allergies of any kind.
Diagnostic Data
His best-corrected entering visual acuities were 20/20 OD and 20/50 OS at distance and near. His external examination was normal for extraocular muscle movement, color, brightness and pupils; however, there was a central abnormality discovered using the facial Amsler during confrontational field testing, OS. Biomicroscopy found normal anterior segment structures and open Van Herick angles with Goldmann applanation tonometry that measured 15mm Hg, OU. The pertinent dilated fundus finding is demonstrated in the photograph.
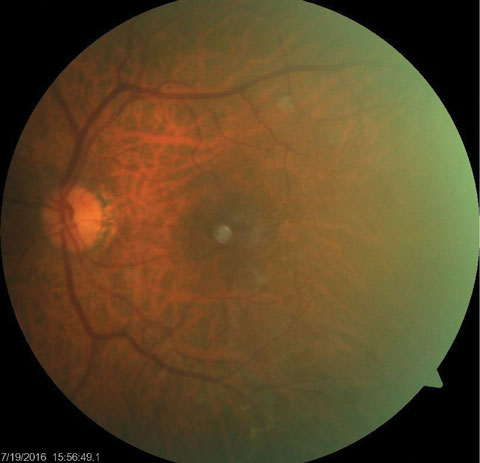 |
| This dilated fundus photograph shows the posterior segment of the left eye of our 65-year-old male patient. He presented complaining of blurry vision in his left eye. Can you diagnose the patient’s condition based on this information? |
Your Diagnosis
How would you approach this case? Does this case require additional tests? How would you manage this patient? What is the likely prognosis?Discussion
Additional studies included fundus phototography to record the lesion and formal Amsler grid testing to provide more precise documentation of the central visual disability and optical coherence tomography (OCT) with deep field tomography to better understand the lesion’s depth and nature.
The diagnosis in this case is pseudovitelliform macular dystrophy in the left eye. Pseudovitellifrom macular dystrophy (PVMD) can be an autosomal dominantly inherited macular disorder or occur sporadically.1-5 PVMD was first described by Gass in 1974.1-5 The overall prevalence of PVMD is unknown.1-5 PVMD has been reported to occur equal between the genders, with a slight preponderance for middle-aged females. Most cases present sometime between the fourth and sixth decade.1,2 One particular study reported a median age of 48 years in females and 61 years in males.1,2
PVMD is characterized by the presence of horizontally oval, yellowish-gray deposits within the macula that are approximately one-fourth to one-half disc diameter in toto.1-5 These lesions appear to be elevated and occurring at the level of the retinal pigment epithelium (RPE).1-5 Some lesions can present in a stellate pattern and are associated with irregular borders. Some lesions possess a reddish halo which is attributed to the contrast between lesion itself and the red-orange color of the surrounding RPE and choroid.1-4,6,7
Patients may be asymptomatic or present with a complaint of visual disruption or metamorphopsia. The most typical complaint is visual acuity decrease noticeable worsening over a period of several months.1 Following the recognition of symptoms most initial presentations demonstrate bilateral asymmetric macular lesions. The few cases that present with unilateral lesions eventually develop a lesion in the fellow eye.1-5,7 Fluorescein angiography (FA) of PVMD reveals a blockage of the choroidal fluorescence (hypofluorescence). Over several years an RPE window defect (hyperfluorescence without leakage) is seen as thr RPE succumbs to the chronic pathophysiology.1-4,7
In 1977, Gass described the phenomenon of basal laminar drusen (BLD) as small subretinal raised yellow lesions that fluoresce and stain during FA producing what he termed the “stars-in-the-sky appearance”.5,6,8 Eight years later light and electron microscopic analysis identified the pathology to the inner portion of Bruch’s membrane with basement membrane-like material originating from the RPE.6 In 1985, Gass reported that BLD were actually focal nodular thickenings of the RPE basement membrane.6 Russell and co-workers disagreed stating that BLD were cellular aggregations of carbohydrates, lipid and protein located between the basal lamina of the RPE and the inner collagenous layer of Bruch’s membrane.6 BLD is associated with increased risk of pseudovitelliform macular dystrophy and its choroidal complications (choroidal neovascularization).6,8 OCT can be helpful in revealing lesions located at or within the RPE or between the RPE and photoreceptor layer.9
In PVMD, an abnormal accumulation of lipofuscin increases RPEphagocytosis. This causes the RPE layer to separate from the photoreceptor layer. RPE disruption is a major contributor to the development and progression of PVMD. The process results in the accumulation of degenerated photoreceptors.5,9,10
Best’s vitelliform dystrophy is the primary differential entity. There are four major differences between Best’s disease and PVMD: age of onset, electro-oculogram responses, evolution of the lesion, and late stage complications.1-3,7 Best’s disease typically presents between the ages of three to 15 with an average of six years.1,5,7 Best’s disease often goes undetected as visual acuity remains good for several years with the atrophic stage occurring around age 40. The electrooculogram (EOG) is normal to slightly abnormal in PVMT. In contrast, the EOG is significantly abnormal in Best’s disease. Although the lesions in both disease processes evolve, PVMD lesions remain fairly stable for several years and show minimal progression in appearance or visual acuity. Best’s disease lesions go through several stages and can result in severe visual impairment. The later stages of Best’s disease can result in a choroidal neovascular membrane (CNV) or sub-retinal hemorrhage while PVMT rarely results in a CNV.1-5,7
Other differentials include pigment epithelial detachment (PED), central serous chorioretinopathy (CSCR), pattern dystrophy and age-related macular degeneration (AMD).1,2,4,5 Early age-related macular degeneration (AMD) can also be confused with PVMT but can be differentiated via fundus examination and OCT.1,2,4,5
Typically, PVMT lesions show minimal progression over a period of several years; retention of good visual function is common. However, the possibility of visual impairment does exist. Impairment in vision is usually associated with the alteration of RPE and resultant macular atrophy.1-5 Rarely, patient’s develop CNV and or macular edema.
PVMD patients should have baseline testing performed including: OCT, visual field, FA, fundus photography, EOG and amsler grid testing with home monitoring to track stability. No treatment is indicated to improve acuity or slow down progression; treatment is only indicated if the disease process progresses to produce choroidal neovascularization or macular edema. Yearly monitoring with measurement of visual acuity should be completed. OCT data should be updated periodically as well. Overall, the majority of these patients maintain good functional vision with a minor decreases over several years.
Dr. Gurwood thanks for Jordan DeMarco, OD his contributions to this case.
|
1. Hodes BL, Feiner LA, Sherman SH, Cunningham D. Progression of pseudovitelliform macular dystrophy. Arch Ophthalmol. 1984;102(3):381-3. 2. Fishman GA, Trimble S, Rabb MF, Fishman M. Pseudovitelliform macular degeneration. Arch Ophthalmol. 1977;95(1):73-6. 3. Glacet-Bernard A, Soubrane G, Coscas G. Macular vitelliform degeneration in adults. Retrospective study of a series of 85 patients. J Fr Ophtalmol. 1990;13(8-9):407-20. 4. Francçois P, Turut P. Vitelliform degeneration of the macula. Arch Ophtalmol Rev Gen Ophtalmol. 1975;35(8-9):609-26. 5. Cherccota V. Pseudovitelliform macular degeneration.Oftalmologia. 2004;48(2):33-6. 6. Meyerle CB, Smith RT, Barbazetto IA, Yannuzzi LA. Autofluorescence of basal laminar drusen. Retina. 2007(8):1101-6. 7. Sabates R, Pruett RC, Hirose T. Pseudovitelliform macular degeneration. Retina. 1982;2(4):197-205. 8. Russell SR, Mullins RF, Schneider BL, Hageman GS. Location, substructure, and composition of basal laminar drusen compared withdrusen associated with aging and age-related macular degeneration.Am J Ophthalmol. 2000;129(2):205-14. 9. Do P, Ferrucci S. Adult-onset foveomacular vitelliform dystrophy. Optometry. 2006;77(4):156-66. 10. Freund KB, Laud K, Lima LH, et. al. Acquired Vitelliform Lesions: Correlation of Clinical Findings and Multiple Imaging Analyses. Retina. 2010: 31(1):13-25. |

