 A 56-year-old black male presented for a consultation. He had been diagnosed with glaucoma six years earlier in Nigeria, but had never received treatment.
A 56-year-old black male presented for a consultation. He had been diagnosed with glaucoma six years earlier in Nigeria, but had never received treatment.
His best-corrected visual acuity was 20/30 O.D. and light perception O.S. His pupils were equal and reactive with a left relative afferent defect. Intraocular pressure measured 30mm Hg O.D. and 23mm Hg O.S.
Biomicroscopy revealed no abnormalities, and gonioscopy demonstrated normal, open, anterior chamber angles. Dilated fundus examination revealed optic disc cupping of 0.75 x 0.75 O.D. and 0.80 x 0.80 O.S.
There was distinct damage to the left neuroretinal rim. Nerve fiber layer analysis via scanning laser polarimetry was abnormal in the left eye; moreover, the remaining neuroretinal rim was pale in comparison to that in the right eye (figures 1 and 2).
Clearly, he had optic atrophy O.S. Furthermore, his visual acuity of light perception was inconsistent with optic disc cupping of 0.80 x 0.80 if glaucoma was the sole cause.
At this point, he was diagnosed with glaucoma as one potential condition and suspected of having an additional neuropathy as well. Threshold perimetry was performed, which revealed a bitemporal visual field defect that respected the vertical hemianopic line in the right eye and crossed over to involve fixation in the left eye (figures 3 and 4).
Bitemporal field loss typically is consistent with a chiasmal lesion, and this was confirmed with magnetic resonance imaging (MRI). This patient had a brain tumor as well as glaucoma.
The key diagnostic findings were disc pallor, visual acuity loss that was much worse than what would be expected from glaucoma, and vertically––rather than horizontally––oriented visual field loss.
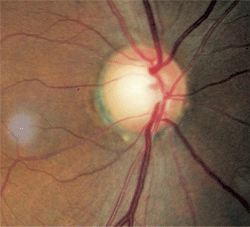
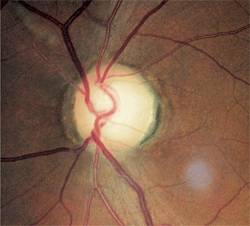
1, 2. Fundus images of our 56-year-old patient. Note the relative disc pallor O.S. (right) compared to O.D. (left).
Diagnosing Glaucoma
Glaucoma is a complex family of diseases in which the level of intraocular pressure, combined with risk factors and other unknown features, causes a characteristic optic neuropathy with associated ganglion cell and nerve fiber layer loss as well as specific types of visual field damage. Correctly diagnosing glaucoma is, in most cases, challenging. When making the diagnosis of glaucoma, consider the disc appearance, nerve fiber layer structure, visual field function and associated risk factors.
It is essential to account for all of these factors when examining patients who are suspected of having glaucoma. Additionally, other conditions, such as compressive tumors of the anterior visual pathway, may give a clinical picture similar to that seen in glaucoma.
So, not only is it crucial to be able to correctly diagnose glaucoma, but you must also be able to differentiate it from other neurological masqueraders. Further, you need to have such other conditions addressed appropriately to enhance the patient’s visual outcome.
Key Differential Findings
When trying to differentiate glaucoma from other neuropathies, such as compressive tumors, there are two rules that you must always remember:
- Pallor in excess of cupping indicates something other than glaucoma. When the rim tissue is pale, suspect some cause other than, or in addition to, glaucoma (figure 5). Rim pallor in glaucoma is rare and is the exception, not the rule. Disc pallor can only be accepted as part of glaucoma when other potential causes have been eliminated.
- Nothing notches a nerve like glaucoma. Focal damage to the neuroretinal rim is very specific to glaucoma. Tumors, inflammation, infection, ischemia or other such pathologies don’t notch a nerve. These conditions may cause increased cupping (often with pallor), but not focal rim notching.
Compressive optic neuropathy occurs by direct impingement of cranial nerve II. Most often, this stems from a space-occupying mass located within the orbit or parasellar area.
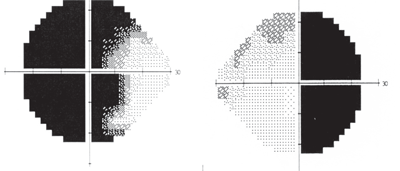
3, 4. Bitemporal visual field defect due to chiasmal tumor (O.D. left, O.S. right).
Conditions that are associated with compressive optic neuropathy include: a dolichoectatic carotid artery; mucocele; thyroid ophthalmopathy; neoplasms, such as optic nerve gliomas, nerve sheath meningiomas, dermoid cysts, neurilemmomas (schwanommas) and orbital metastases; vascular anomalies, such as cavernous hemangioma, lymphangioma, simple venous varix, arteriovenous malformation, carotid aneurysm and carotid cavernous fistula; inflammations, such as orbital cellulitis and orbital pseudotumor; Wegener’s granulomatosis; and subperiosteal or intraorbital hemorrhage.1-8
Additionally, intracranial parasellar lesions, such as pituitary adenoma and craniopharyngioma, can produce the same effect. In these cases, the patient will likely have vertically oriented visual field loss.9,10
Neuroimaging and Glaucoma
In 1998, David Greenfield, M.D., and associates published a seminal paper that discussed which glaucoma patients required neuroimaging.11
The authors compared glaucoma patients who had normal intraocular pressure and had undergone neuroimaging as part of their evaluation (due to the normal IOP) with patients in whom compressive lesions were diagnosed. They found that none of the patients diagnosed with normal-tension glaucoma had neuroradiologic evidence of a mass lesion in the anterior visual pathway.
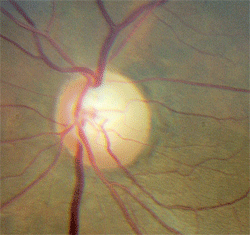
5. Superior rim notching and pallor—characteristic of glaucoma—that must be evaluated with neuroimaging.
In contrast, patients with mass lesions of the anterior visual pathway typically had visual acuity less than 20/40, vertically-aligned visual field defects, optic disc pallor in excess of cupping and were less than 50 years old.
The authors concluded that younger age, poorer visual acuity, vertically-aligned visual field defects and neuroretinal rim pallor were more indicative of a compressive mass lesion than glaucoma.
Be aware of the features that differentiate optic nerve compression from glaucoma, and learn to recognize when patients may have something other than, or even in addition to, glaucoma. As our mentor in neuro-ophthalmic disease, the late Dr. Larry Gray taught us: “Always remember to pull the ‘–omas’ out of glaucoma.”
1. Rosen N, Ben Simon GJ. Orbital decompression in thyroid related orbitopathy. Pediatr Endocrinol Rev. 2010 Mar;7(Suppl 2):217-21.
2. Oono S, Kurimoto T, Fukazawa K, Mimura O. Compressive optic neuropathy caused by a paranasal sinus cyst of Wegener’s granulomatosis. Jpn J Ophthalmol. 2007 Nov-Dec;51(6):480-1.
3. Chua CN, Alhady M, Ngo CT, et al. Solitary nasal neurofibroma presenting as compressive optic neuropathy. Eye (Lond). 2006 Dec;20(12):1406-8.
4. Mansour AM, Salti HI. Multiple myeloma presenting with optic nerve compression. Eye (Lond). 2001 Dec;15(Pt 6):802-4.
5. Wu W, Sun MT, Cannon PS, et al. Recovery of visual function in a patient with an Onodi Cell Mucocele compressive optic neuropathy who had a 5-week interval between onset and surgical intervention: A Case Report. J Ophthalmol. 2010;2010:483056. Epub 2010 Oct 12.
6. George JL, Marchal JC. Orbital tumors in children: clinical examination, imaging, specific progression. Neurochirurgie. 2010 Apr-Jun;56(2-3):244-8.
7. Aakalu VK, Ahmad AZ. Wegener granulomatosis causing compressive optic neuropathy in a child. Ophthal Plast Reconstr Surg. 2009 Jul-Aug;25(4):327-8.
8. Jacobson DM. Symptomatic compression of the optic nerve by the carotid artery: clinical profile of 18 patients with 24 affected eyes identified by magnetic resonance imaging. Ophthalmology. 1999 Oct;106(10):1994-2004.
9. Stark KL, Kaufman B, Lee BC, et al. Visual recovery after a year of craniopharyngioma-related amaurosis: report of a nine-year-old child and a review of pathophysiologic mechanisms. J AAPOS. 1999 Jun;3(6):366-71.
10. Drummond SR, Weir C. Chiasmal compression misdiagnosed as normal-tension glaucoma: can we avoid the pitfalls? Int Ophthalmol. 2010 Apr;30(2):215-9.
11. Greenfield DS, Siatkowski RM, Glaser JS, et al. The cupped disc: Who needs neuroimaging? Ophthalmology. 1998 Oct;105(10):1866-74.

