Glaucoma is a significant public health problem but represents an opportunity for doctors to make a positive impact in long-term eye care. In Part 1 last month, we outlined some of the reasons why an OD would pursue a glaucoma-focused clinic. New technologies and a renaissance of established techniques provide doctors with an incredible arsenal of testing to comprehensively care for patients, to prevent vision loss and quality of life reduction arising from glaucoma.
In Part 2, we take a step into the consulting room and describe the diagnostic and management processes involved in glaucoma.
The Diagnosis Paradigm
The process of identifying glaucoma is complex, with no single cut-off criterion for diagnosis, unlike other lesion-based diagnoses like age-related macular degeneration and diabetic retinopathy. Although glaucoma is the most common optic neuropathy, it shares several features with other conditions that also manifest with visual field (VF) defects, loss of neural tissue or changes at the optic nerve. Glaucoma remains a diagnosis of exclusion when assessing suspected optic nerve head disease. Careful inspection of the nerve and surrounding features can help differentiate these conditions.
 |
|
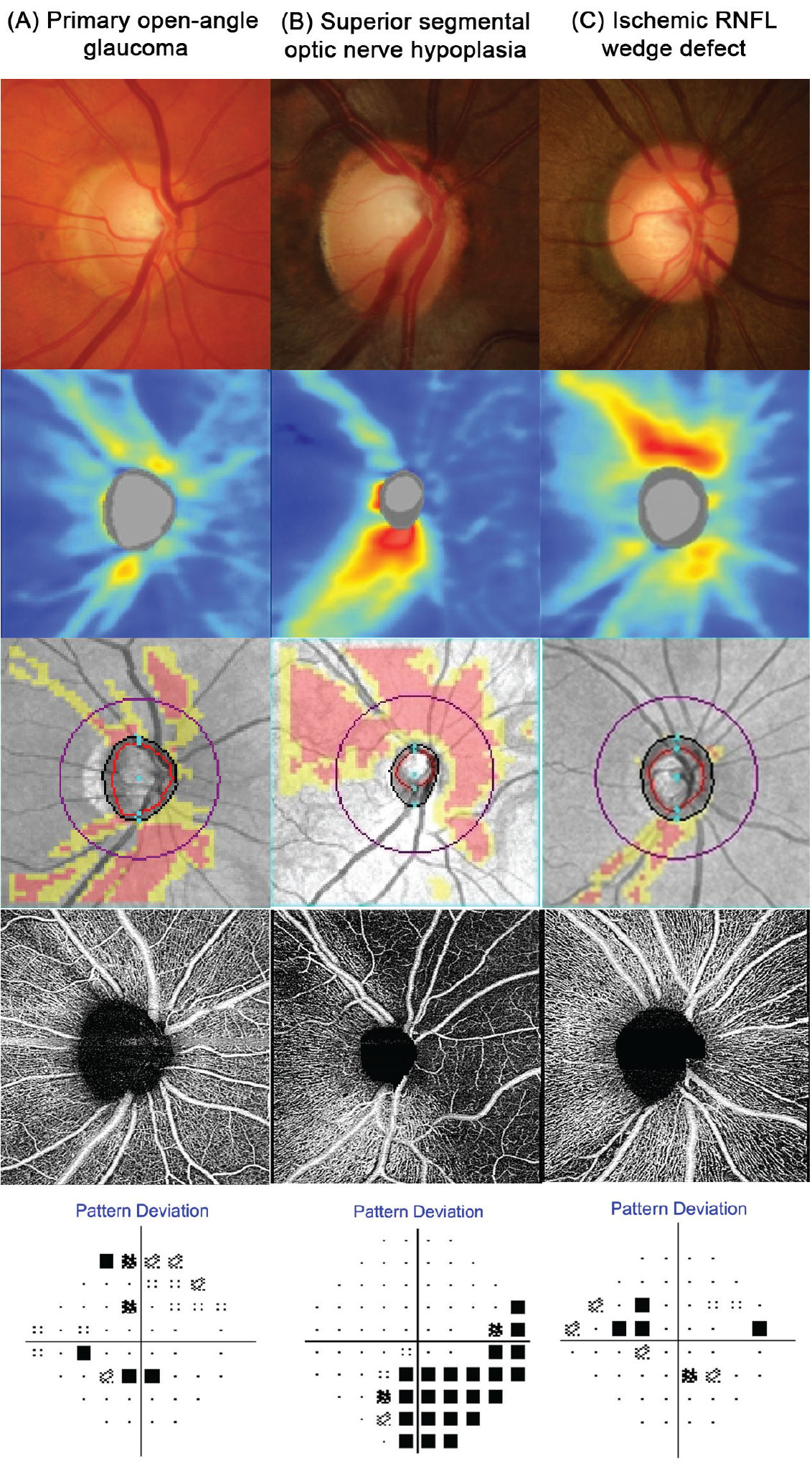
Fig. 1. Funduscopic and OCT findings for three contrasting patients: (A) A patient with classic primary open-angle glaucoma. This disc appears cupped with a gradient of retinal nerve fiber layer loss and a shallow reduction in perfusion on OCT-A. (B) A patient with superior segmental optic nerve hypoplasia. This disc has a sharply delineated absence of neural tissue in the superior rim, with a marked loss of nerve fiber layer, perfusion and visual function. The sectoral nature of the loss in addition to the attenuation and absence of retinal vasculature is indicative of a congenital vascular anomaly rather than glaucoma. (C) A patient with an ischemic retinal nerve fiber layer defect. Unlike in patient A, the neuroretinal rim appears intact and is thus incompatible with glaucoma. Supplementary imaging using OCT-A confirms a very deep and focal loss of perfusion that is not typical in early glaucoma as implied by the shallow VF defect and little neural loss. Click image to enlarge. |
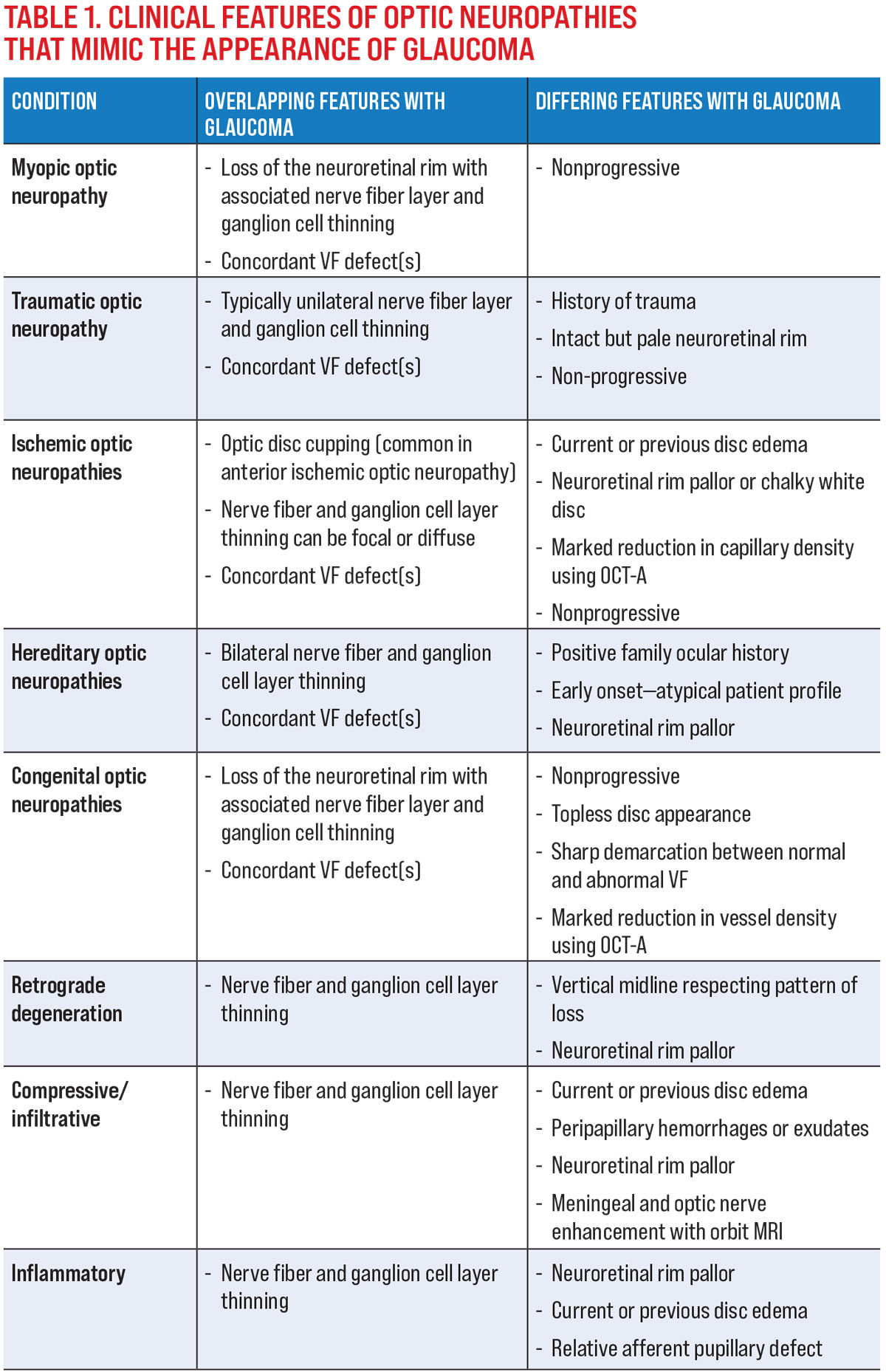
The common mimickers of glaucoma and their clinical features that overlap with or differentiate each entity from glaucoma are shown in Table 1.
To aid this process, we recommend four key considerations in the differential diagnosis of glaucoma:
1. Patient profile, ocular and medical history. For example, consider a history of trauma in the context of a pale but intact neuroretinal rim with neural tissue thinning and a VF defect, which points toward a traumatic optic neuropathy rather than glaucoma. Similarly, the patient’s age is also helpful, as several optic neuropathies mimicking glaucoma often manifest in younger patients and may point clinicians toward a hereditary or congenital cause instead.
2. Longitudinal data. Glaucoma is a progressive optic neuropathy; thus documented stability in the presence of untreated structural or functional loss precludes glaucoma as a diagnosis. In cases such as myopic optic neuropathy where there is significant overlap in the clinical presentation, longitudinal data is key for differentiating between these disease entities and can prevent unnecessary treatment.1
3. “Characteristic” changes to the optic nerve and peripapillary region. A defining feature of glaucoma is presence of neuroretinal rim thinning. As such, loss of neural tissue (e.g., nerve fiber layer thinning) in the absence of visible optic nerve head changes points towards a non-glaucomatous etiology.2 Pallor of the nerve head is also not a classic feature of glaucoma and warrants investigation into other causes of optic atrophy. These signs should signal the need for further neuro-ophthalmic assessment, such as color vision.
Optical coherence tomography angiography (OCT-A) is also useful in evaluating non-glaucomatous optic neuropathies, as it can highlight vascular changes that are not visible using traditional OCT or funduscopically. Although glaucoma has been shown to result in a reduction in OCT-A parameters such as vessel density, this typically does not occur until later in the disease process. A marked reduction in vessel density in the presence of shallow neuroretinal rim loss points to an ischemic or congenital condition rather than glaucoma (Figure 1).
4. The pattern of structural and functional loss. Retrograde degeneration presents with classic patterns of both structural and functional loss, which allow for retinotopic mapping to the location of insult. Carefully scrutinize patients presenting with midline respecting structural or functional loss for a non-glaucomatous etiology and consider neuroimaging to investigate other diseases of the visual pathway.3
 |
| Click image to enlarge. |
Paradigm shifts in glaucoma diagnosis have arisen with the changing availability of specific devices or techniques such as fast VF testing and progression analysis, high resolution structural examination and intraocular pressure (IOP) profiling. Improved understanding of the multifactorial pathophysiology of glaucoma has also underscored the need to perform other non-ocular assessments to rule out contributions from systemic and non-ocular comorbidities.
Personalized Medicine
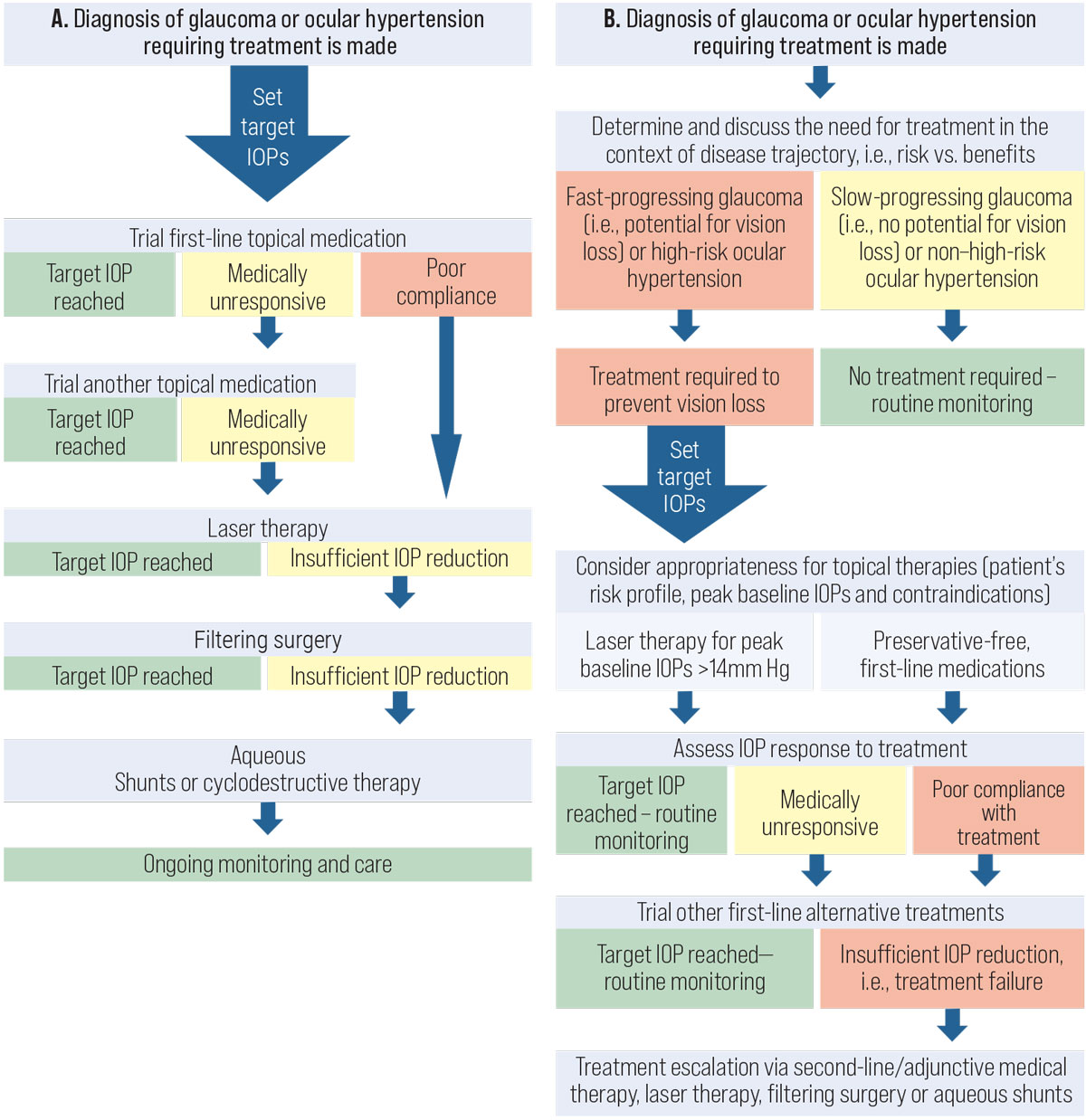
After a diagnosis is made, the doctor should then develop a management plan with the patient and the rest of the health care team.4 A commonly deployed treatment algorithm in the past is shown in Figure 2A, which demonstrates the concept of treatment escalation with increasingly invasive IOP-lowering therapies. A more recent stepwise algorithm that we have suggested is shown in Figure 2B. In the updated algorithm, several significant changes to clinical care are highlighted:
- Determining the need for treatment.
- Preservative-free drops as primary therapy.
- Primary laser (or non-drop) therapy.
- Emerging surgical options as first-line treatment.
Does the patient even require treatment? One of the biggest changes in recent years is the concept of the need for therapy. The trajectory of glaucoma differs for each patient. The seminal Early Manifest Glaucoma Trial gave clinicians important information about the natural history of glaucoma.5 From those results we know that pseudoexfoliative glaucoma tends to progress the quickest, followed by high-tension glaucoma and then normal-tension glaucoma as the slowest. However, even within the separate groups of glaucoma subtypes, there is significant variability. Therefore, it is incumbent on the clinician to determine the trajectory of their individual patient.
The importance of disease trajectory lies in the fundamental reasons for treating glaucoma: to preserve vision and maintain quality of life. In cases where the patient’s disease trajectory is not likely to significantly affect their quality of life, any activated treatment may conversely lead to a reduction in quality of life. Other considerations include the costs of treatment. In such cases, there may be a reason to monitor without treatment to mitigate these issues.
 |
|
Fig. 2. (A) The current framework used in the management of patients with glaucoma. (B) Our new proposed framework for the management of patients with glaucoma. Click image to enlarge. |
Preserved or non-preserved? Traditionally, topical therapy has been the mainstay, first-line treatment for glaucoma, with prostaglandin analogs preferred due to their convenient once-daily dosing and favorable safety profile.
However, benzalkonium chloride in glaucoma drugs is known to detrimentally affect the ocular surface, especially with long-term use.6,7 Preservative-free formulations have been suggested as an alternative. Recent studies have shown that preservative-free medications are similarly effective.8-10 Therefore, clinicians should weigh the potential advantages and disadvantages of both formulations before proceeding.
SLT as first-line. In suitable patients, selective laser trabeculoplasty (SLT) may be a viable first-line treatment that addresses fundamental issues with topical therapy such as compliance and adverse effects.11 While the benefits of SLT are clear, doctors should remember that, in patients with IOP lower than 21mm Hg (i.e., normal-tension glaucoma), the amount of IOP reduction achieved using SLT was lower. Some studies have shown that at pressure levels below 15mm Hg, the probability of success is less than 30%.12-14
Compared with medical therapy, SLT runs the risk of being an episodic point-of-care that may lead to patient non-adherence to follow-up.15 Remind patients of the importance of review appointments, even if everything seems fine from their perspective.
Referrals and Communication
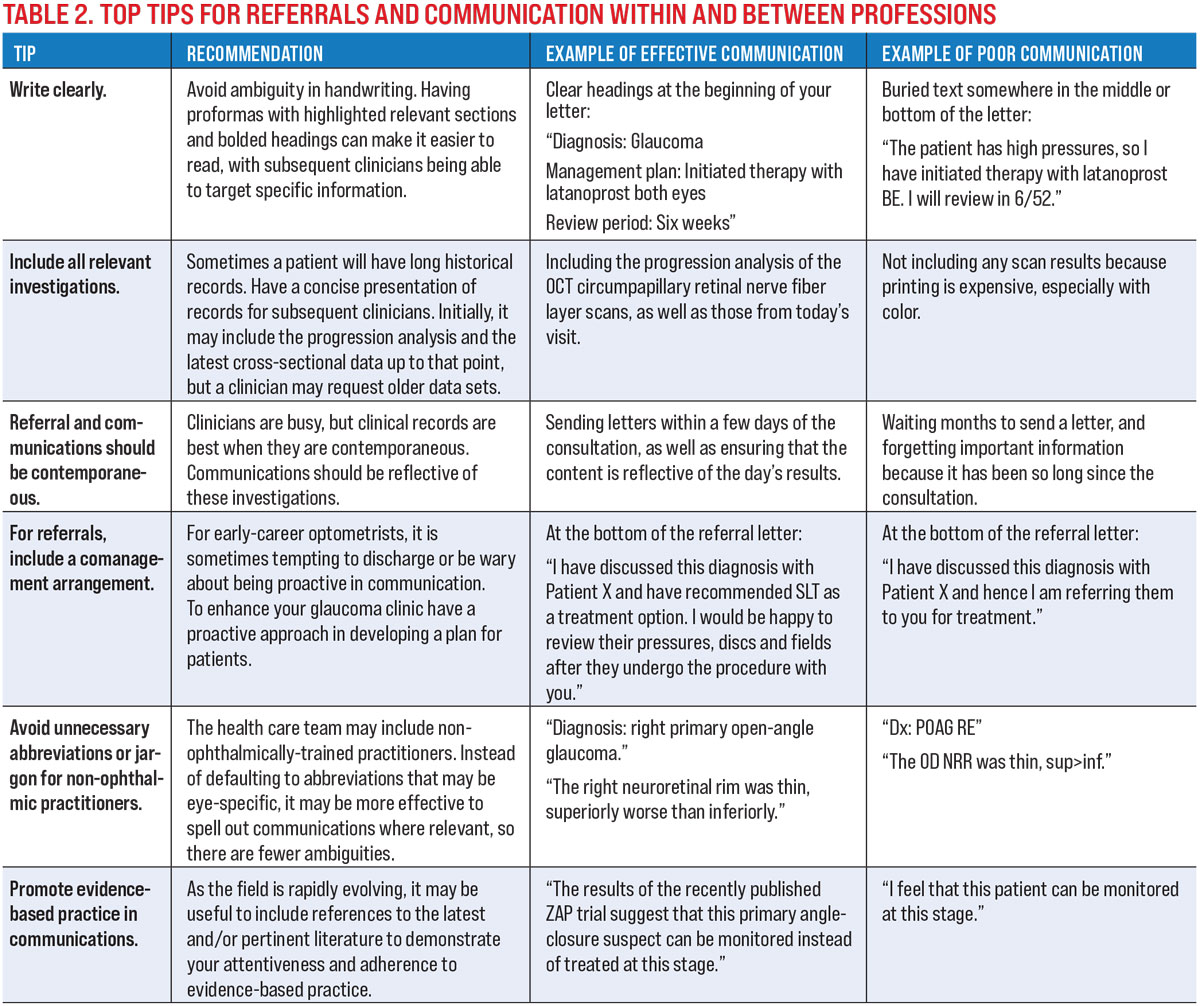
A successful glaucoma practice involves effective communication intra-professionally and inter-professionally.16,17 Clinicians who are just starting off may find it useful to obtain feedback from their optometry or ophthalmology peers, especially with unusual presentations or complex cases.18 Establishing a glaucoma clinic requires forging relationships with glaucoma specialists and understanding the services that they may offer. Even the patient’s primary care physician requires regular updates on the patient’s ocular status.
Our top tips for referrals and communications within and between professions are shown in Table 2.
 |
| Click image to enlarge. |
Monitoring Visits
Typically, review periods may range from three months to one year, depending on the individual’s risk of progression.19-21 Rubrics for assessing risk or risk calculators (such as from the Ocular Hypertension Treatment Study) are accessible, but remember that individuals do not necessarily fall into the typical mold.22 Examples of considerations for clinicians in risk titration include:
Demographics: Age, ethnicity and gender may not match the clinical trial or guideline that you are deploying.
Financial constraints: Are patients able to financially afford frequent review visits?
Travel/distance constraints: Are patients able to practically attend appointments during typical work hours?
Clinic workflow and capacity constraints: Does the clinic have the capacity to review these patients on a more frequent basis?
Risk of glaucoma-related consequences: What is the patient’s risk of vision loss and impairment of quality of life? Where does this stand relative to other factors such as morbidity and mortality?
Medication prescriptions: Typically, six-month review periods are recommended in many clinics due to the need for repeat prescriptions—however, can this be done in other ways?
The goal of these follow-up visits is to identify significant disease progression. There needs to be a balance between the anticipated diagnostic yield (more likely to see the “right patient at the right time”) and having potentially wasteful and costly review appointments.
Follow-up Consultation
Tests that identify markers of progression should include both structural and functional components, as patients may show progression on either or both indices.23,24 Be prepared to take an updated medical history to identify contributory risk factors for glaucoma. These may include new diagnoses of other concurrent systemic disease, new meds and newly discovered family history of glaucoma.
IOP measured at follow-up visits may provide additional data regarding the patient’s IOP profile. Fluctuations or significant elevations may signal the need for closer monitoring or earlier intervention.
Guidelines for repeating gonioscopy or anterior chamber angle assessment also provide a broad range of recommendations. It is fairly well-established that the anterior chamber angle narrows with age, increasing the risk of angle closure.25 Pseudoexfoliation, the most common identifiable cause of secondary open-angle glaucoma, also has a tendency to increase in prevalence with age.26 Since it is unlikely that these issues manifest within a short time frame, it would be appropriate to repeat gonioscopy about every two years, with routine screening at visits in between.
Be vigilant for any changes that may signal the need for treatment plan modifications. Examples could include:
Unreliable VF results at the last visit: The key is to identify repeatable VF progression at the next visit.
A disc hemorrhage at the previous visit: The next visit will specifically target the identification of consequent structural or functional loss.
IOP was not quite meeting target, but there was no structural or functional progression: The next visit may be to reassess to see if target pressure is met.
Progression Analysis
An advantage of quantitative assessment techniques such as standard automated perimetry and OCT is the ability to quantify changes and perform regression analyses to identify statistically significant progression over time.
Although quantitative values are a useful indicator to identify trends, as with all similar measurements there are sources of variability that may confound the final result. Many clinicians complain about VF testing for being highly subjective and therefore variable. Sources of variability include intra-session fatigue, inter-session learning and the patient’s mental state.27,28 However, although OCT may be more objective than perimetry, it also has sources of variability that manifest in the form of artifacts: either instrument-related (internal noise), acquisition-related or patient-related.29
Aside from these sources of variability, spacing out tests and monitoring of a patient over a long period of time introduces aging to the progression analysis. The clinician needs to be able to identify a significant change that overcomes the normal age-related decline in both VF sensitivity and neural thickness. One strategy is to use the output rate of change and put it in context of the patient’s age: at this rate of progression, will the patient likely lose significant vision in their lifetime, and when might this happen?
Aside from quantitative analyses of VF sensitivity and retinal thickness data, comparisons of serial fundus photos may also be useful for qualitative identification of structural change. Fundus photography may also reveal the presence or absence of a disc hemorrhage.
Whilst disc hemorrhages have been considered to be a marker for glaucoma progression, disc hemorrhages have been recognized to occur spontaneously or as part of the natural history of glaucoma, not necessarily representing a more aggressive course of the disease.30 Thus, these patients require more monitoring for identifying realized structural and functional loss.
Remember, structural and functional examination results indicate the endpoints for glaucoma. IOP control is best described as a predictor for risk of future progression. IOP reductions that have not met target, or are in isolation, are not indicators of disease progression, but they must be considered in the context of structure and function. In some cases, over-aggression of IOP control may not be required in a stable glaucoma patient.
Similar to diagnosis, progression analysis requires a combinatory approach to identify the “tipping point” at which a patient converts from a glaucoma suspect to manifest glaucoma, or is identified as a glaucoma progressor. Unfortunately, identification of progression may be as much an art as it is a science. Often, a single test within a suite is insufficient to convince a clinician that a change in treatment plan is required, especially when that test is known to have variable results. Several research groups have suggested that changes occurring in glaucoma may appear in different tests, depending on the stage of the disease.31 Broadly, in early glaucoma OCT has been suggested to be superior to perimetry, and in late glaucoma perimetry, especially 10-2, may be more useful. However, there is an overall lack of consensus regarding what constitutes significant change.
However, if a clinician considers that the goal of glaucoma treatment is to prevent irreversible blindness and impairment to quality of life, there may be a rationale for emphasizing perimetric changes over structural loss. This philosophy stems from the notion that VF results are better reflective of the patient’s functional vision and quality of life, and thus represents the “real-world” impact of the disease.
In the present clinical environment, how might one confidently identify perimetric loss, given its notoriety for variability? Recent developments in automated perimetry have targeted more efficient ways of testing, with strategies such as reduced test time, scotoma targeting and seeding with structural data.32-34 Some of these strategies have arrived in clinical practice, and fast testing methods allow clinicians to perform more examinations per visit, thereby overcoming the variability arising from having fewer data points.
Changing Management Strategy
Once change has been satisfactorily identified using the tools available in your office, you might wonder what the next step for the patient is. Many authoritative guidelines have provided guidance on management algorithms. For example, a typical flowchart approach might begin with either a first-line topical medication or SLT; treatment escalation would be followed by topical medications in a step-wise approach of intensity, before arriving at surgical intervention.
Clinicians need to be aware of the side effect profiles of each treatment paradigm, as the decision to escalate should involve a balancing of the potential threat to vision against detriments to quality of life potentially arising from more intense therapy.
Takeaways
In this two-part article, we have provided an overview of the steps required for the beginner to start a glaucoma clinic. Hopefully, this has demonstrated that whilst there are a lot of considerations in glaucoma, it is also potentially intellectually stimulating. Most importantly, the doctor becomes an integral caregiver for the patient, who in turn can become a long-term, loyal attendee of the practice.
Dr. Phu is a clinician-scientist with academic and clinical positions at the Centre for Eye Health in Kensington, New South Wales, Australia, and the School of Optometry and Vision Science, University of New South Wales. He is a Fellow of the American Academy of Optometry and a Diplomate in glaucoma.
Dr. Wang is a research and clinical staff optometrist at the Centre for Eye Health. She is a Fellow of the AAO. They have no financial interests to disclose.
1. Hsu CH, Chen RI, Lin SC. Myopia and glaucoma: sorting out the difference. Curr Opin Ophthalmol. 2015;26(2):90-5. 2. Leaney JC, Nguyen V, Miranda E, et al. Bruch’s membrane opening minimum rim width provides objective differentiation between glaucoma and nonglaucomatous optic neuropathies. Am J Ophthalmol. 2020;218:164-72. 3. Zangerl B, Whatham A, Kim J, et al. Reconciling visual field defects and retinal nerve fiber layer asymmetric patterns in retrograde degeneration: an extended case series. Clin Exp Optom. 2017;100(3):214-26. 4. Phu J, Agar A, Wang H, et al. Management of open-angle glaucoma by primary eye-care practitioners: toward a personalised medicine approach. Clin Exp Optom. 2020. 5. Heijl A, Bengtsson B, Hyman L, et al. Natural history of open-angle glaucoma. Ophthalmology. 2009;116(12):2271-6. 6. Fogagnolo P, Torregrossa G, Tranchina L, et al. Tear film osmolarity, ocular surface disease and glaucoma: a review. Curr Med Chem. 2019;26(22):4241-52. 7. Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: the PESO study. J Glaucoma. 2013;22(9):730-5. 8. Aptel F, Pfeiffer N, Schmickler S, et al. Noninferiority of preservative-free vs. BAK-preserved latanoprost-timolol fixed combination eye drops in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2019;28(6):498-506. 9. Aptel F, Choudhry R, Stalmans I. Preservative-free vs. preserved latanoprost eye drops in patients with open-angle glaucoma or ocular hypertension. Curr Med Res Opin. 2016;32(8):1457-63. 10. Day DG, Walters TR, Schwartz GF, et al. Bimatoprost 0.03% preservative-free ophthalmic solution versus bimatoprost 0.03% ophthalmic solution (Lumigan) for glaucoma or ocular hypertension: a 12-week, randomised, double-masked trial. Br J Ophthalmol. 2013;97(8):989-93. 11. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505-16. 12. Pillunat KR, Spoerl E, Elfes G, Pillunat LE. Preoperative intraocular pressure as a predictor of selective laser trabeculoplasty efficacy. Acta Ophthalmol. 2016;94(7):692-6. 13. Mao AJ, Pan XJ, McIlraith I, et al. Development of a prediction rule to estimate the probability of acceptable intraocular pressure reduction after selective laser trabeculoplasty in open-angle glaucoma and ocular hypertension. J Glaucoma. 2008;17(6):449-54. 14. Martow E, Hutnik CM, Mao A. SLT and adjunctive medical therapy: a prediction rule analysis. J Glaucoma. 2011;20(4):266-70. 15. Clement CI. Initial treatment: prostaglandin analog or selective laser trabeculoplasty. J Curr Glaucoma Pract. 2012;6(3):99-103. 16. Cheng J, Beltran-Agullo L, Trope GE, Buys YM. Assessment of the quality of glaucoma referral letters based on a survey of glaucoma specialists and a glaucoma guideline. Ophthalmology. 2014;121(1):126-33. 17. Tobin-Schnittger P, O’Doherty J, O’Connor R, O’Regan A. Improving quality of referral letters from primary to secondary care: a literature review and discussion paper. Prim Health Care Res Dev. 2018;19(3):211-22. 18. Gagliardi A. Use of referral reply letters for continuing medical education: a review. J Contin Educ Health Prof. 2002;22(4):222-9. 19. National Health and Medical Research Council. Guidelines for the screening, prognosis, diagnosis, management and prevention of glaucoma. Internet: Commonwealth of Australia, 2010. 20. National Institute for Health and Care Excellence. Glaucoma: diagnosis and management. London, UK: National Institute for Health and Care Excellence; 2019. 21. Prum BE, Jr., Lim MC, Mansberger SL, et al. Primary open-angle glaucoma suspect preferred practice pattern guidelines. Ophthalmology. 2016;123(1):P112-51. 22. Ocular Hypertension Treatment Study G, European Glaucoma Prevention Study G, Gordon MO, et al. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114(1):10-9. 23. Medeiros FA. Biomarkers and Surrogate Endpoints: Lessons learned from glaucoma. Invest Ophthalmol Vis Sci. 2017;58(6):BIO20-6. 24. Ohnell H, Heijl A, Brenner L, et al. Structural and functional progression in the early manifest glaucoma trial. Ophthalmology. 2016;123(6):1173-80. 25. Phu J, Tong J, Zangerl B, et al. Cluster analysis reveals patterns of age-related change in anterior chamber depth for gender and ethnicity: clinical implications. Ophthalmic Physiol Opt. 2020;40(5):632-49. 26. Ekstrom C, Winblad von Walter L. Incidence and baseline risk factors for pseudoexfoliation in Sweden: a long-term follow-up study. Acta Ophthalmol. 2020;98(3):310-4. 27. Marra G, Flammer J. The learning and fatigue effect in automated perimetry. Graefes Arch Clin Exp Ophthalmol. 1991;229(6):501-4. 28. Hudson C, Wild JM, O’Neill EC. Fatigue effects during a single session of automated static threshold perimetry. Invest Ophthalmol Vis Sci. 1994;35(1):268-80. 29. Li A, Thompson AC, Asrani S. Impact of artifacts from optical coherence tomography retinal nerve fiber layer and macula scans on detection of glaucoma progression. Am J Ophthalmol. 2021;221:235-45. 30. Kim KE, Park KH. Optic disc hemorrhage in glaucoma: pathophysiology and prognostic significance. Curr Opin Ophthalmol. 2017;28(2):105-12. 31. Medeiros FA, Zangwill LM, Bowd C, et al. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. 2012;53(11):6939-46. 32. Turpin A, Morgan WH, McKendrick AM. Improving Spatial Resolution and Test Times of Visual Field Testing Using ARREST. Transl Vis Sci Technol. 2018;7(5):35. 33. Chong LX, Turpin A, McKendrick AM. Targeted spatial sampling using GOANNA improves detection of visual field progression. Ophthalmic Physiol Opt. 2015;35(2):155-69. 34. Phu J, Khuu SK, Agar A, Kalloniatis M. Clinical Evaluation of Swedish interactive thresholding algorithm-faster compared with swedish interactive thresholding algorithm-standard in normal subjects, glaucoma suspects and patients with glaucoma. Am J Ophthalmol. 2019;208:251-64. |

