 |
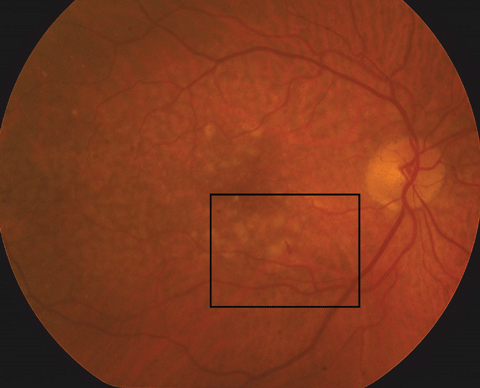
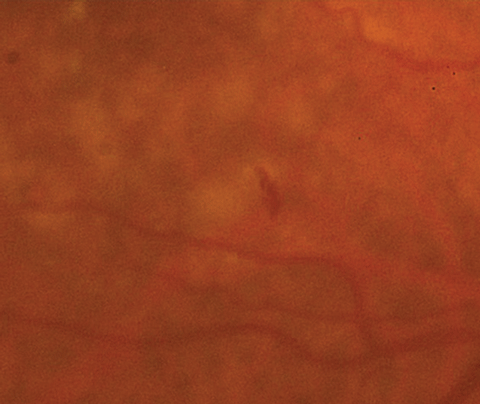
| Figs. 1a and 1b. At left, fundus photo of the patient’s right eye. Below, a close- up shot of the above fundus photo shows a small, juxtafoveal intraretinal hemorrhage of the right eye. Click images to enlarge. |
 |
An 81-year-old Caucasian male presented for a six-month follow up for dry age-related macular degeneration (AMD). He had been monitored every six months with a dilated eye examination and ocular coherence tomography (OCT) starting two years prior. There were no visual complaints and he had not noted any changes with Amsler grid testing once a week at home.
History
His overall medical history was unremarkable except for a history of osteoporosis. He was taking 50,000 units vitamin D, aspirin 81mg, omega-3 1200mg, and Preservision AREDS twice daily. He had no known drug allergies. He did not smoke at the time of presentation, but had previously been a smoker starting in his late teenage years for about five years.
Examination
His distance visual acuity in both eyes was 20/30 best corrected with no improvement using pinhole. His pupillary function, confrontation visual fields and extraocular motilities were normal in each eye. His intraocular pressure was 12mm Hg OU.
Anterior segment evaluation was significant for mild meibomian gland dysfunction, dry eyes and 2 to 3+ nuclear sclerotic cataracts. Posteriorly, he did have a vitreous detachment in each eye. Optic nerves were healthy in both eyes, with small cup-to-disc ratios and peripapillary atrophy OS. The macula of the left eye had many large, soft drusen with retinal pigment epithelial (RPE) changes sans fluid or elevation. In the macula of the right eye, similar large drusen and RPE changes were noted, but there was also a small intraretinal hemorrhage present inferior to the fovea (Figure 1a and 1b).
OCT angiography (OCTA) (Figures 2 and 3) and intravenous fluorescein angiography (IVFA) (Figure 4) were obtained and are available for review.
Take the Quiz
1. Based on the number and size of drusen, what is the classification of AMD in the left eye?
a. Early dry.
b. Intermediate dry.
c. Advanced dry.
d. Exudative.
2. What do you see in the right eye’s fluorescein angiography?
a. Dark choroid.
b. Two microaneurysms inferior to fovea.
c. Hyperfluorescing drusen only.
d. Two hyperfluorescing lesions with mild late leakage inferior to fovea.
3. What is the likely diagnosis?
a. Retinal angiomatous proliferation with AMD.
b. Dry AMD alone.
c. Retinal macroaneurysms with AMD.
d. Central serous chorioretinopathy with AMD.
4. What does the OCTA reveal?
a. Leakage of vessels.
b. Subretinal abnormal vascular proliferation.
c. Outer retinal abnormal vascular proliferation.
d. Intraretinal abnormal vascular proliferation.
5. What should our management strategy be?
a. Monitor q6mo with dilated exam, continue AREDS and Amsler.
b. Monitor q3mo with dilated exam, continue AREDS and Amsler.
c. Refer to retina specialist.
d. Refer to retina specialist, but only once vision is decreased.
Discussion
Our patient was being followed on a regular schedule for his AMD. Historically, it had been dry in both eyes, so he was taking appropriate AREDS2 formula supplements and self-monitoring with an Amsler grid between visits. On a routine visit, an intraretinal hemorrhage noted in the right eye prompted additional testing.
The OCTs of the fovea of each eye revealed drusen without associated subretinal fluid. When the scan pattern was aligned atop the area of retinal thickening inferior to the fovea OD, there was what appeared to be a small serous pigment epithelial detachment (PED) with disruption of overlying inner retinal architecture. The OCTA revealed vessel proliferation within the sensory retina extending to the outer retina in the area of macular thickening and hemorrhage (Figure 2).
Though on OCT, the space underlying the PED appeared transparent and not fibrous or drusenoid, an IVFA was done to rule out a choroidal neovascular membrane (CNVM). The IVFA was consistent with hyperfluorescence staining of drusen in both eyes and mild late leakage of two focal areas inferior to the fovea in the right eye. There was no occult leakage seen, and it was not suggestive of any CNVMs.
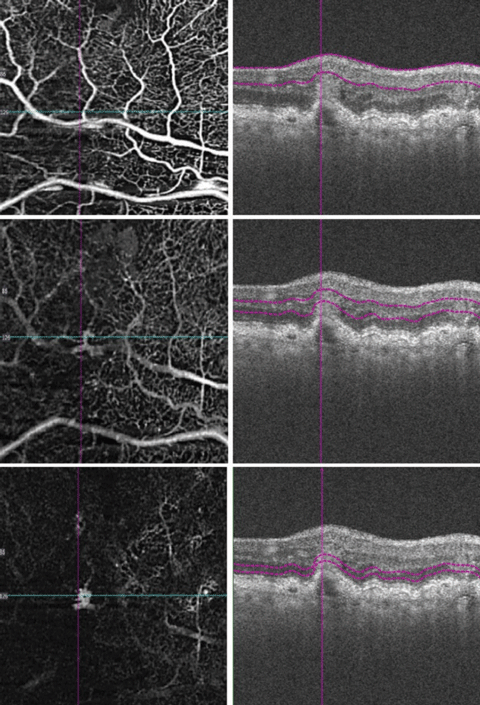 |
| Fig. 2. OCT angiography images segmenting the retinal layers from anterior to posterior. |
Our patient had a focal serous PED that correlated with OCTA findings of abnormal vasculature in the outer retina, which should be avascular (Figure 2, bottom image). The OCTA colored depth-encoded map (Figure 3) signifies vessel depth by a variation in the color spectrum; reds and oranges denote inner vasculature whereas blues and purples denote deeper vessels. Note, OCTA does not image leakage itself, but rather captures serial B-scans in a given location to map volumetric flow changes. These flow changes represent erythrocyte movement through blood vessels and vascular nets.
Diagnosis
Our patient was diagnosed with retinal angiomatous proliferation (RAP). RAP lesions—initially described in 1992, but updated in 2001—are defined as intraretinal vascular abnormalities which form and then dive deeper to form anastomoses with choroidal vasculature.1,2 They tend to leak and may cause subretinal fluid in the form of serous PEDs, which one study found present in 22% of RAP patients.3 Some controversy still exists as to the exact pathogenesis of these lesions.4
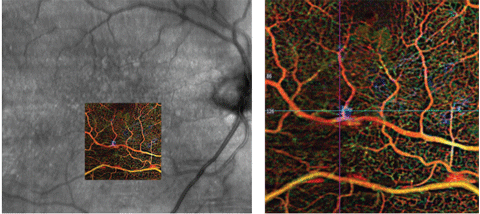 |
| Fig. 3. OCT angiography en face photo with color- coded map representing vessel depth. Click image to enlarge. |
Retinal angiomatous proliferation lesions are commonly associated with AMD and represent progression of disease. One study shows 7.3% of patients with AMD had RAP lesions, but this number is likely even higher.1 The lesions are typically found bilaterally and juxtafoveally, and since they represent conversion to neovascular AMD, they require referral to a retinal specialist for treatment.5
Treatment and management of this specific subset of AMD is varied, but may include laser photocoagulation, photodynamic therapy, intravitreal steroid or intravitreal anti-VEGF. Individual therapies may also be combined in some cases. There has yet to be a prospective, comparative study for treatment, but RAP lesions in AMD are typically more refractory to treatment and often require more injections.6
Our patient was promptly referred to a retinal specialist, and he received an intravitreal injection of Avastin (bevacizumab, Genentech). Upon his one month follow up, the intraretinal hemorrhage had absorbed and he was given another shot of Avastin as part of a treat-and-extend protocol. n
This case was contributed by Alison Bozung, OD, optometric resident at Bascom Palmer Eye Institute.
|
1. Hartnett ME, Weiter JJ, Gardts A, Jalkh AE. Classification of retinal pigment epithelial detachments associated withdrusen. Graefes Arch Clin Exp Ophthalmol. 1992; 230:11–19. 2. Yannuzzi, LA, Negrao S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter J, Freund KB, Sorenson J, Orlock D, Borodoker N. Retinal Angiomatous proliferation in age-related macular degeneration. Retina. 2001; 21: 416-34. 3. Slakter JS, Yannuzzi LA, Schneider U, et al. Retinal choroidal anastomoses and occult choroidal neovascularization in age-related macular degeneration. Ophthalmology 2000; 107:742–54. 4. Scott AW, Bressler SB. Retinal angiomatous proliferation or retinal anastomosis to the lesion. Eye. 2010; 24: 491-6. 5. Yannuzzi, LA. 2003. Retinal Angiomatous Proliferation in AMD. Review of Ophthalmology. 3 (25). Retrieved from http://www.reviewofophthalmology.com/content/d/retinal_insider/i/1341/c/25684/. 6. Engelbert M, Zweifel SA, Freund KB. ‘‘Treat and extend’’ dosing of intravitreal antivascular endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. Retina. 2009; 29: 1424–31. |
Retina Quiz Answers:
1) b; 2) d; 3) a; 4) d; 5) c.

