 A 62-year-old white male presented with a chief complaint of blurred vision, which he believed was due to cataracts. He reported a steady, bilateral decrease in vision (O.S. > O.D.) over the past two years.
A 62-year-old white male presented with a chief complaint of blurred vision, which he believed was due to cataracts. He reported a steady, bilateral decrease in vision (O.S. > O.D.) over the past two years.
His medical history was significant for type 2 diabetes; hypertension; peripheral artery disease; a single basal cell carcinoma and a single squamous cell carcinoma, which had both been excised the previous year. His current medications included 1,000mg metformin q.d., 81mg aspirin q.d., 1,000mg Niaspan (niacin, Abbott) q.d., 1g Lovaza (omega-3-acid ethyl esters, GlaxoSmithKline) q.d., 20mg simvastatin q.d., 5mg amlodipine q.d. and 25mg Hyzaar (losartan potassium-hydrochlorothiazide, Merck) q.d.
The patient noted an allergy to both lobster and conch. Additionally, he reported a 10-pack per year history of smoking (he quit in 1975), and suggested that he currently drinks three beers a day.
On examination, his visual acuity measured 20/40 O.D. and 20/70 O.S. with habitual correction. His best-corrected visual acuity was 20/20 O.D. and 20/30 O.S. He had a 10-prism diopter constant alternating exotropia. Confrontation fields were full to careful finger counting, and extraocular motility was full.
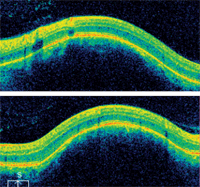
Horizontal (top) and vertical SD-OCT scans of the patient’s left macula. Are all the retinal layers intact?
The patient’s pupils were equal, round and reactive to light, without afferent defect. The anterior segment examination was normal, except for grade 1+ nuclear sclerotic cataracts O.U.
On dilated fundus exam, the vitreous was clear. The optic nerves appeared healthy, with small cups, distinct margins and good rim coloration and perfusion. Retinal vasculature was normal. The remainder of the fundus exam was normal in the right eye. In the left eye, however, we noted an elevated area with several drusen located just temporally off the optic disc. The peripheral retinal findings in both eyes were unremarkable.
Take the Retina Quiz
1. What does the OCT show?
a. Pigment epithelial detachment (PED).
b. Choroidal mass.
c. Choroidal neovascular
membrane (CNVM).
d. Posterior staphyloma.
2. What are the essential findings of the A-scan?
a. It shows nothing unusual.
b. It shows a low-reflective choroidal lesion.
c. It shows a high-reflective choroidal lesion.
d. It shows a high-reflective retinal lesion.
3. What is the correct diagnosis?
a. Choroidal melanoma.
b. Central serous retinopathy (CSR) secondary to niacin intake.
c. Metastatic lesion.
d. Choroidal hemangioma.
4. How should this patient be managed?
a. Observation.
b. Photodynamic therapy (PDT).
c. Intravitreal anti-VEGF injection.
d. Panretinal photocoagulation (PRP).
For answers, see
below.
Discussion
On clinical examination, we observed several drusen in the area located just superior temporal to the optic nerve. On indirect binocular ophthalmoscopy, this area appeared vaguely elevated and was a bright red to orange color, which can be seen in the fundus photograph. This was unique to the remainder of the background retinal appearance. The elevated area appeared to approach the macula; however, there was no serous retinal detachment present. Based on this clinical appearance, we were highly suspicious that the patient had a choroidal hemangioma. We ordered an OCT scan as well as ultrasound testing to help confirm the diagnosis.
The elevated area of the patient’s left eye could almost have been mistaken for a retinal pigment epithelium (RPE) detachment. However, RPE detachments tend to be more sharply defined than what was seen in this case. In our patient, the borders were poorly defined and the elevation was subtler. This indicated that the elevation evolved from the choroid and involved the RPE and retina. Indeed, this was confirmed on OCT, which showed a dome-shaped elevation at the level of the choroid that pushed the neurosensory retina and RPE anteriorly. Also of note, there was no fluid accumulation in any of the retinal layers.
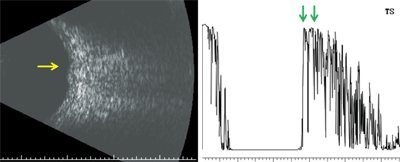
Here are the results of our patient’s ultrasound (B-scan left, A-scan right).
B-scan ultrasonography revealed a vascularized, dome-shaped mass that measured 5mm x 4.5mm x 1.1mm. Interestingly, while performing the scan, the echo signal changed rapidly within the lesion, which was suggestive of blood flow. Observing vascularity within a lesion is a dynamic process that cannot be appreciated in a single-scan image. The diagnostic A-scan showed that the mass was located at the level of the choroid and exhibited high internal reflectivity. There was no evidence of extraocular extension, so we knew the mass was confined to the choroid.
The classic clinical appearance of this lesion––along with OCT and ultrasound––confirmed the diagnosis of circumscribed choroidal hemangioma.
A circumscribed choroidal hemangioma is a benign vascular tumor of the choroid that is composed of blood vessels of varying size. The tumors usually are round or oval, slightly elevated, and have a subtle orange-red coloration, which makes them difficult to distinguish from the surrounding choroid. Typical dimensions of these lesions are 6mm of base diameter and 3mm of thickness.
The thickness of the tumor in our patient measured roughly 1mm, which created a subtle elevation and indistinct borders that were most easily seen on indirect binocular ophthalmoscopy. Nearly all such tumors are located within the parapapillary and paramacular areas, and do not extend anterior to the equator unless they are extremely large.1,2
Circumscribed choroidal hemangiomas have no known systemic disease association. This presentation should not be confused with diffuse choroidal hemangioma, which often presents in children and is associated with Sturge-Weber syndrome. Additionally, diffuse choroidal hemangiomas exhibit a markedly dissimilar clinical appearance and require a different management strategy.
Most patients with circumscribed choroidal hemangiomas are asymptomatic, and these lesions are likely to be discovered as an incidental finding during fundus examination. If patients become symptomatic, it is often around the age of 40, as the tumor can enlarge over many years. Visual changes depend on the location of the hemangioma as well as the presence of overlying retinal fibrosis and atrophy.1
Historically, the diagnosis of these lesions often was made on pathology––when the eyes were enucleated––and was based on a high suspicion of malignancy. However, with current technology, it is now much easier to identify this benign tumor.
When evaluating a suspected choroidal hemangioma, ultrasound provides the greatest diagnostic value and is key for differentiating this tumor from a potentially life-threatening condition, such as choroidal melanoma. Choroidal hemangiomas and choroidal nevi (as another example) show high internal reflectivity, while melanomas show low internal reflectivity. Vascularity does not help to differentiate them, because malignant tumors are often highly vascularized; however, a benign vascular tumor will also exhibit this characteristic.
Evaluating the lesion depth on B-scan ultrasonography is of interest as well. In our patient, we observed no extraocular extension. However, in some malignant cases, there is destruction of surrounding tissue and the mass penetrates into the deeper structures.
The OCT does not have the same diagnostic value as ultrasound. However, it is important to obtain an OCT to look for retinal or subretinal fluid accumulation, which could be an indication of potential growth and/or the need for treatment. Moreover, in most practices, the OCT likely is the easiest and most reliable test to initially differentiate a subtle choroidal mass from subretinal fluid or a PED.
Most patients with a choroidal hemangioma do not require treatment unless they become symptomatic secondary to foveal involvement, the presence of subretinal fluid or the development of a retinal detachment. If intervention is required, the current standard of care is anti-VEGF therapy or PDT.3,4
Given the lack of macular fluid and his reasonably good vision, we elected to observe our patient. There is no question that the lesion had an architectural effect on the nearby macula; however, we didn’t believe that any form of treatment would change that.
So, we reassured our patient about the findings and gave him a prescription for glasses. We asked him to return in six months––or sooner, should he develop additional symptoms.
Thanks to Sara McGowan, O.D., optometry resident at the Bascom Palmer Eye Institute in Miami, for contributing this case.
1. Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976 May-Jun;20(6):415-31
2. Shields CL, Honavar SG, Shields JA, et al. Circumscribed choroidal hemangioma: Clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001 Dec;108(12):2237-48.
3. Tonuzi A. Photodynamic Therapy in Subfoveal Choroidal Hemangioma. Retina Today: Retinal Oncology. 2011 Mar;73-5.
4. Sagong M, Lee J, Chang W. Application of intravitreal bevacizumab for circumscribed choroidal hemangioma. Korean J Ophthalmol. 2009 Jun;23(2):127-31.
Answers to the Quiz
1. b
2. c
3. d
4. a

