Ptosis, formally known as blepharoptosis, is a common finding characterized by upper eyelid drooping in primary gaze. Commonly, ptosis is merely a reality of aging, but some occurrences are related to systemic diseases or genetic disorders. While cosmesis may be the primary concern for some individuals with ptosis, more advanced cases are associated with visual field disruption, eyelid strain, altered head position in an effort to compensate and headaches due to forehead and scalp muscle strain.1 All this can decrease a patient’s quality of life.
Here, we describe some of the causes of ptosis, with an emphasis on acquired forms, and will offer guidelines on monitoring and working up patients.
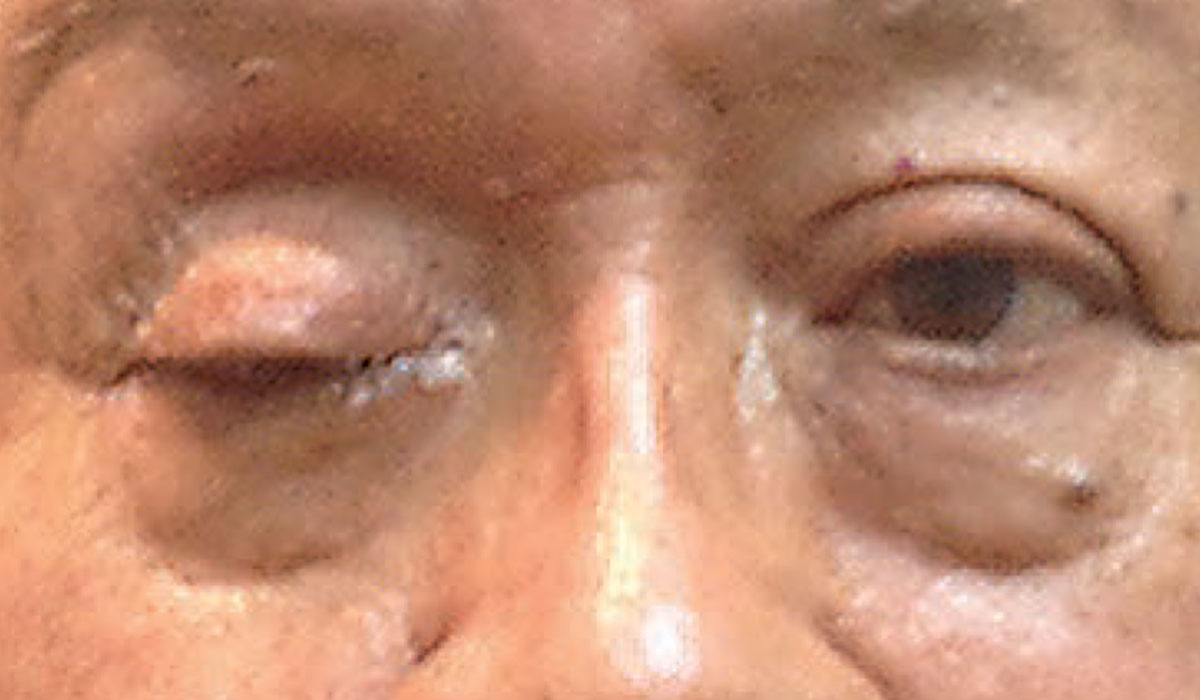 |
| Complete ptosis could indicate the presence of an emergent neurological condition. Photo: Michael Trottini, OD. Click image to enlarge. |
Anatomical Explanation
Mechanically, ptosis is linked to dysfunction of the muscles responsible for eyelid elevation. These are the superior palpebral levator and the superior tarsal muscle, also called Müller’s muscle. Loss of tonus in either of them results in ptosis.2 However, the superior palpebral levator is the main retractor of the upper eyelid and therefore deficiency in its function produces a more significant ptosis.2 The levator originates from the lesser wing of the sphenoid bone and becomes a fan-shaped tendinous expansion, the levator aponeurosis, as it enters the eyelid.2 The fibers of the aponeurosis penetrate the orbital septum and extend into the upper lid, fanning out across its entire length and insert on the anterior aspect of the tarsal plate. The levator receives its innervation from the superior division of the cranial nerve III (CN III).3
Müller’s muscle originates from the inferior aspect of the levator, just posterior to the fornix, and inserts on the superior edge of the tarsal plate.4
This muscle is innervated by sympathetic fibers; denervation of Müller’s muscle will cause only a mild ptosis of 1mm to 2mm.3-5
Patient Evaluation
When an individual presents with complaints of ptosis, the first step is to obtain a thorough clinical history. This should include a careful review of past systemic and ocular medical history, any changes in health and medication use.
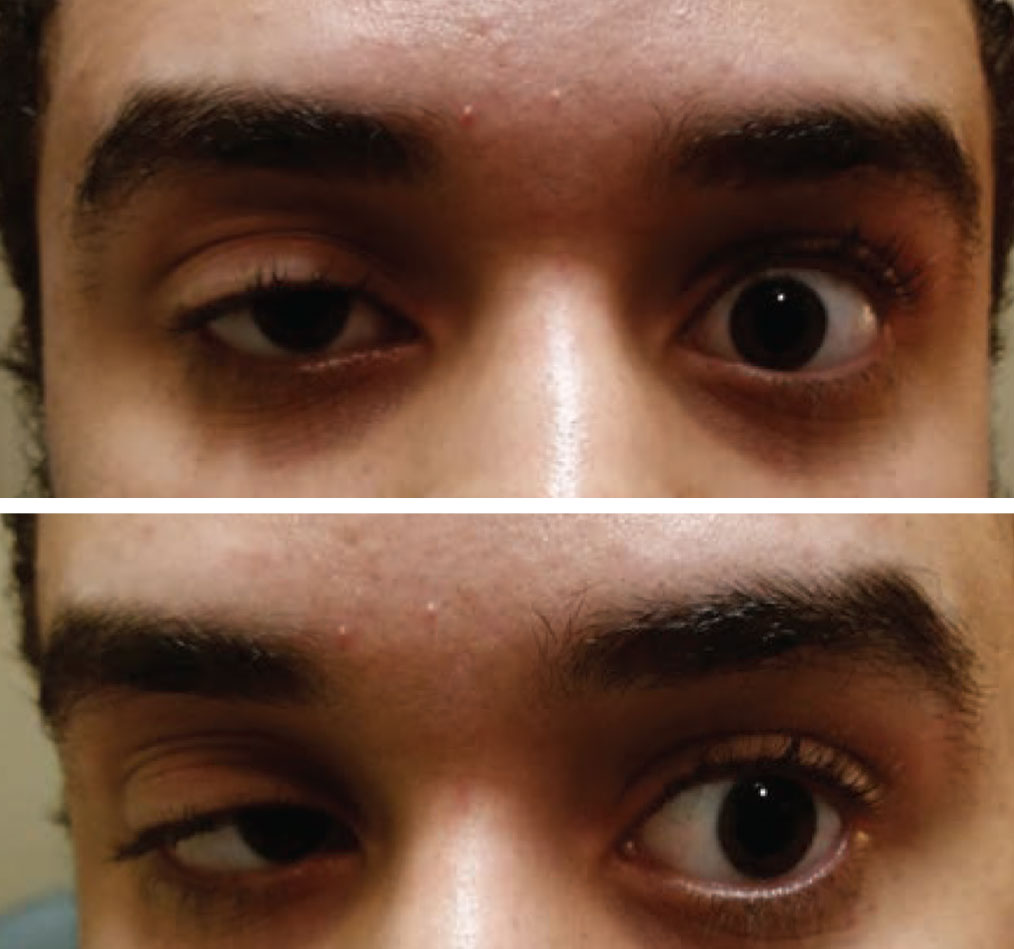 |
| Acquired ptosis in one eye and a motility restriction in the other should be considered myasthenia gravis until proven otherwise. Photo: Paul Ajamian, OD. Click image to enlarge. |
Ptosis can be either congenital or acquired, monocular or binocular and progressive or non-progressive. These characteristics should be noted in the case history along with an inquiry of any associated neurologic and ophthalmic symptoms, including blurred vision, diplopia, pain, peripheral field loss, headaches or generalized muscle weakness. Inquire about any trauma or past ocular surgeries, along with a history of past botulinum toxin injections or ophthalmic steroid use.6,7
Understanding the course with regard to duration of the ptosis and whether it is progressing, resolving or unchanged from onset is important along with any indication of diurnal variability. An acute onset of hours or days prior to examination raises concern for serious pathologic etiology.
Reviewing old photographs can always help confirm any change if the patient is a poor historian.8
Clinical Examination
Evaluating patients with ptosis incorporates many of the standard components of a comprehensive eye exam. Visual acuity and binocular vision assessment can aid in indicating a possible causative systemic disease as well as in assessing for amblyopia or misalignment. If patients were to undergo surgical correction of the ptosis, amblyopia or phorias could indicate potential fusion issues. Pupil and extraocular motility testing along with external observation for globe asymmetry are critical to evaluate for other related pathology discussed later. Observing the patient’s head position and visual field testing can help determine the impact and severity of the ptosis.
Quantitative measurements of eyelid position aid in assessing retractor muscle function, identifying anatomical abnormalities and can also be helpful to monitor for progression or improvement of ptosis over time.
Primarily, these measurements, called margin reflex distance (MRD) 1, 2 and 3, are performed using a penlight directed at the patient’s eyes while in primary gaze. For MRD1, the distance in millimeters between the central portion of the upper eyelid margin and the pupillary reflex is recorded for both eyes.9-11 A normal MRD1 is 4mm to 5mm.5 The difference in MRD1 between the normal and the ptotic eye is the amount of ptosis. If the ptosis is significant enough that the pupillary reflex cannot be visualized, then the distance in millimeters that the eyelid must be raised until the pupillary reflex can be seen is recorded as the MRD1 in negative numbers.9
MRD2 differs in that it measures in the primary gaze, from the corneal light reflex to the central portion of the lower lid.12 MRD3 determines how much levator to resect in patients with congenital ptosis, who have a vertical strabismus associated with ptosis and in whom strabismus surgery is not indicated.12
Levator function can be measured by determining the total movement in millimeters of the upper eyelid from downgaze to upgaze. This measurement is important for the surgeon when determining the most appropriate corrective procedure. Normal levator function is typically greater than 15mm, while 12mm to 14mm is considered good, 5mm to 11mm is considered fair and anything less that 4mm is poor.5
The upper eyelid crease represents the junction of the levator aponeurosis to overlying orbicularis muscle. The area from the upper eyelid margin in downgaze to the lid crease normally measures approximately 8mm in men and 9mm to 10mm in women, although it can vary by race.10 In patients with congenital and myogenic ptosis, the upper eyelid crease is often absent or subtle. While in patients with aponeurotic ptosis, the upper eyelid crease is positioned much higher.11
The palpebral fissure is simply the widest distance between the upper and lower eyelid margins in primary gaze and provides an overall comparison of relative ptosis. Average values are between 8mm and 11mm.10
Acquired Ptosis Classification
After performing an exam, the next step is to classify your patient’s presentation. Based on etiology, ptosis can be classified as aponeurotic, myogenic, neurogenic, mechanical or traumatic. However, they may also have something called pseudoptosis, which describes an eyelid that appears ptotic due to structural changes that indirectly affect lid position.
Aponeurotic ptosis is the most common acquired ptosis. Typically seen in older individuals, it can present at any age as a result of trauma, frequent eye rubbing or prolonged use of contact lenses with hard lens wearers at higher risk.6 Levator function is generally good in these patients, but the levator aponeurosis is stretched or thinned due to repetitive stress and effects of gravity and aging.6 Younger patients exhibiting this form of acquired ptosis may display a higher eyelid crease due to levator disinsertion.13 These patients generally respond well to surgical correction.
Mechanical ptosis can be caused by any abnormality that weighs down or alters the structure of the lid, including blepharochalasis, cellulitis, hordeolum, orbital fat prolapse and lid or orbital tumors. Scarring from inflammation, surgery, Stevens-Johnson syndrome or ocular pemphigoid can also lead to mechanical ptosis.6 Always perform orbital imaging in patients with an underlying mass or infiltrative lesion. Addressing the causative factors is the initial management strategy in these patients.
Traumatic ptosis can develop as the result of any trauma to the orbit. The causes can include disinsertion of the levator muscle or damage to the levator tendon with scar formation. Alternatively, CN III damage can also sustain damage leading to the ptosis.6,14 In severe cases that result in significant damage to the levator, patients may require multiple surgeries with a poor probability of restoring natural eyelid symmetry.6,14 Patients who are at increased risk for CN III involvement include those with head injuries, post-traumatic cavernous sinus thrombosis, orbital apex fractures and nerve compression by foreign bodies.14 Patients with CN III damage will typically resolve on their own with time and should be observed for spontaneous recovery over a period of three to six months before considering surgical intervention.6,14
Pseudoptosis can result from numerous conditions, including blepharospasm, dermatochalasis with hooding, brow ptosis, microphthalmos, enophthalmos and phthisis bulbi.8 Lid retraction in the contralateral eye, as in thyroid eye disease, can also cause a relative ptotic appearance to the unaffected eye.8
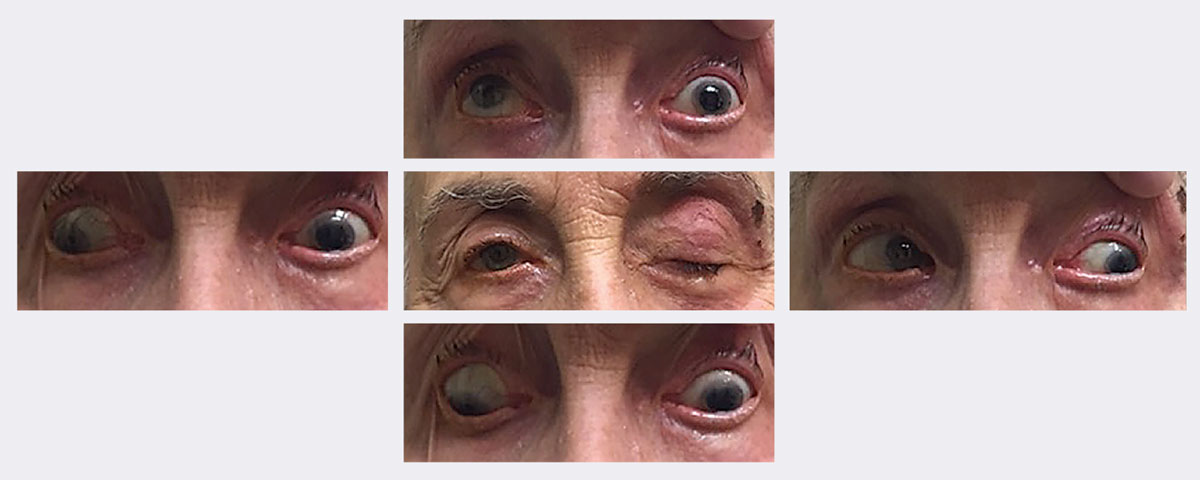 |
| At this patient’s initial visit, she had ptosis with restricted upgaze, downgaze and adduction. She was eventually diagnosed with a complete, pupil-sparing left 3rd nerve palsy secondary to herpes zoster. She resolved in approximately two weeks using an antiviral. Photos: Michael Trottini, OD. Click image to enlarge. |
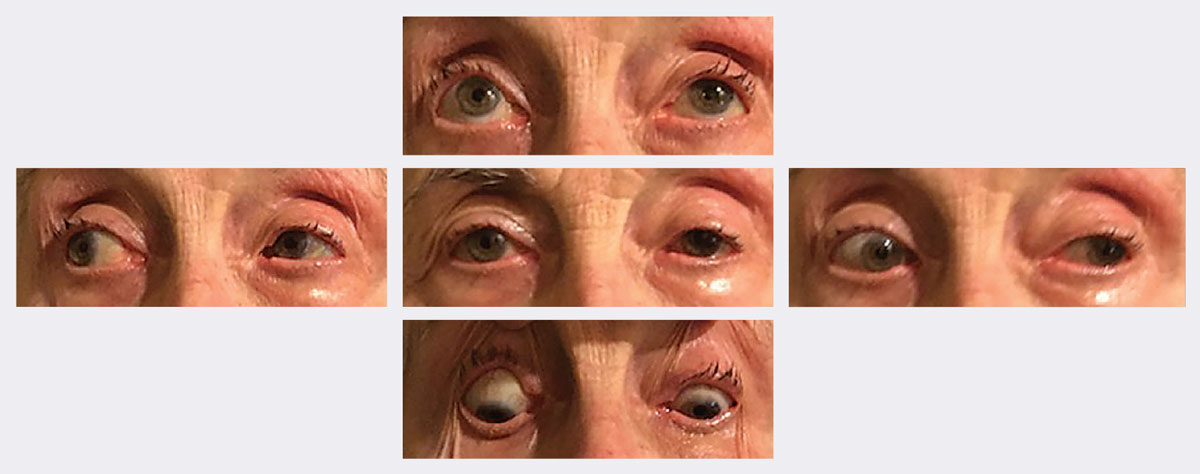 |
| At follow-up, her ptosis and motilities significantly improved in two weeks. Photos: Michael Trottini, OD. Click image to enlarge. |
Neurogenic Ptosis
This decreased innervation to the muscles of the upper eyelid can stem from a number of potential etiologies, some of which can be life threatening. Prompt identification and management of these conditions are critical.
Third nerve palsy is the most common cause of neurogenic ptosis.1 This nerve innervates the superior, inferior and medial rectus muscles, the inferior oblique, the levator, and the pupillary sphincter muscle.15 When ptosis is accompanied by symptoms of diplopia or pupillary mydriasis, a third nerve palsy must be considered.
Third nerve palsies cause the eye to assume a “down-and-out” position with significant weakness of elevation, depression and adduction on motility testing.16 The degree of severity of a third nerve palsy can vary greatly depending on the cause and anatomical location of the lesion.15
Ptosis associated with a third nerve palsy may be partial or complete. Patients presenting with an incomplete third nerve palsy in which there is only partial paresis of the extraocular muscles or only partial ptosis should be evaluated very closely for progression to complete palsy with pupillary mydriasis. Pupillary involvement associated with a third nerve palsy is concerning for a compressive lesion such as an aneurysm or neoplasm.
In patients older than 50, the most common etiology of third nerve palsy is microvascular ischemia in the presence of cardiovascular risk factors, followed by aneurysm, neoplasm, trauma, and various inflammatory and infectious etiologies.16 Patients with third nerve palsy and pupillary involvement need to be sent for urgent neuroimaging of the head to rule out life threatening aneurysm, particularly aneurysm of the posterior communicating artery.15,16
Horner syndrome is characterized by unilateral ptosis, pupillary miosis and facial anhidrosis secondary to interruption of sympathetic innervation to the eye. The ptosis associated with Horner syndrome is mild, typically only 1mm to 2 mm, and is due to lack of innervation to Müeller’s muscle in the upper eyelid. Ptosis in Horner syndrome can be variable and may even be absent in up to 12% of cases. Miosis occurs due to loss of sympathetic tone of the pupillary dilator muscle. The resulting anisocoria is more pronounced in the dark and a dilation lag is often evident within the first five seconds of dark exposure.17
Horner syndrome can result from a lesion anywhere along the three-neuron oculosympathetic pathway. Confirmation can be made through pharmacological testing with 0.5% apraclonidine or, less commonly, cocaine.17,18 A positive apraclonidine test will result in dilation of the miotic pupil due to hypersensitivity of the iris dilator muscle as well as normalization of lid position.1,17
Hydroxyamphetamine can be used to determine if a lesion is pre- or postganglionic; however, this is rarely used in modern practice since neuroimaging will typically be ordered regardless.17,18
Myasthenia Gravis TestingTwo tests that can easily be performed in the exam room to evaluate for myasthenia gravis are the rest and ice tests. For the rest test, patients with ptosis are told to keep their eyes gently closed for two minutes. Approximately 50% of patients with myasthenia gravis will show an improvement in ptosis of 2mm or more with the rest test.1 Researchers believe this happens because the amount of ACh within the synapse accumulates, improving muscle response. The ice test is performed by placing ice on the ptotic eyelid for two minutes and then re-evaluating the ptosis. Like the rest test, an improvement of 2mm or more is a positive test result.1,2 Ptosis secondary to other causes will not improve with either the rest or ice tests.1 Lab testing for AChR antibodies should also be performed in suspected cases of myasthenia. Keep in mind that anywhere from 30% to 65% of patients with purely ocular myasthenia gravis will be seronegative.3,4 Studies show the level of AChR antibodies does not correlate with severity of disease or aid in prediction of who will develop generalized disease.3,5 The Tensilon test has long been regarded as the primary diagnostic test for myasthenia gravis, though today it is used less frequently than in the past due to risk of cardiac complications, syncope and cholinergic crisis. It also has a high incidence of false negatives and false positives.1
|
Emergent imaging for adults is not necessary unless Horner syndrome presents acutely with pain. Recent studies argue that in patients with new-onset isolated Horner syndrome and no localizing signs or symptoms, imaging should focus on the entire oculosympathetic pathway using MRI and angiography since potential etiologies are so numerous. A causative lesion is typically identified in 20% of cases, the most common being carotid artery dissection, which can occur spontaneously or secondary to trauma.
Other serious etiologies that must be ruled out are malignancy, vascular lesions of the brainstem, and cavernous sinus thrombosis.1,17,18 In cases of longstanding Horner syndrome, typically considered two years or longer, imaging may not be warranted unless new symptoms develop.17
Myasthenia gravis is a rare disorder characterized by an antibody mediated immune attack on the nicotinic acetylcholine receptor (AChR).19 In up to 65% of patients, the initial signs and symptoms of myasthenia gravis include ptosis, extraocular muscle weakness, weakness of the orbicularis oculi or ocular misalignment.20 All symptoms can present unilaterally or bilaterally and tend to be recurrent and variable with progressive worsening with fatigue. Typically, more than 90% of patients will experience some or all of these symptoms to some degree during the course of the disease.21,22 Purely ocular myasthenia gravis occurs in approximately 50%.21 Approximately half will progress to generalized disease within two years.23-25
Any patients with confirmed or even suspected myasthenia should also undergo CT imaging in order to evaluate for thymic hyperplasia or thymoma.21 Thymoma occurs in approximately 10% to 15% of patients with myasthenia and requires surgical resection.26
Myogenic Ptosis
This most common congenital ptosis can be either acquired or congenital and is due to dysgenesis of the levator muscle with fibroadipose tissue found in place of skeletal muscle fibers.6 Acquired myogenic ptosis describes a rare form of typically bilateral progressive ptosis, caused by systemic muscular dysfunctions.6,27,28 This can include muscular dystrophy, myasthenia gravis (discussed previously), oculopharyngeal dystrophy and chronic progressive external ophthalmoplegia (CPEO).6 Less commonly, steroid-induced and HAART-associated myogenic ptosis have been reported.27,28
Myotonic dystrophy is the most common form of adult-onset muscular dystrophy. Ocular findings include symmetrical ptosis, blepharospasm, ophthalmoplegia, and Christmas tree cataracts. Myotonia (delayed relaxation of skeletal muscle after contraction) is often the initial symptom; thus, affected individuals may be diagnosed prior to onset of ocular symptoms. Affected patients may also present with early-onset frontal alopecia, wasting of temporal and masseter muscles and distal limb weakness exacerbated by cold, excitement or fatigue.29,30 Respiratory disease and cardiac myopathy are the most common causes of death.30
Myogenic ptosis may be corrected with an eyelid crutch or surgery; however, use caution when recommending either of these, as these patients have a higher risk of postoperative dry eye disease and exposure keratopathy due to poor Bell’s phenomenon and incomplete blink.31,32
When a patient presents with ptosis, a thorough case history and clinical exam are critical in determining when further work-up or referral is necessary as ptosis may be the initial presenting symptom of a number of potentially fatal medical conditions.
As a general clinical pearl, any time ptosis, pupillary anomaly, extraocular motility dysfunction, globe asymmetry or dystopia is detected on clinical exam, a thorough evaluation of each is warranted as they may give clues to concurrent disease.8,33 Depending on the etiology, some cases of ptosis will resolve spontaneously, others will require management of systemic disease, and some will require surgery. As with any procedure, postsurgical complications can occur, including, but not limited to undercorrection, overcorrection resulting in lagophthalmos and dry eye, eyelid crease abnormalities and distortion of the lid margin.
Optometrists must have an awareness of potential etiologies and the ability to discern when ptosis presentation is an emergent situation requiring prompt diagnostic testing, treatment, and referral.
Drs. Reinhard and Spampinato are optometrists at the Cincinnati Veteran’s Administration Medical Center and adjunct faculty members at the Ohio State University College of Optometry.
1. Díaz-Manera J, Luna S, Roig C. Ocular ptosis: differential diagnosis and treatment. Curr Opin Neurol. 2018;31(5):618-627. 2. Oyster CW. The Human Eye: Structure and Function. Sunderland, MA: Sinauer Associates; 2006. 3. Remington, L.A. Clinical Anatomy of the Visual System (Second Edition). Butterworth-Heinemann; 2005. 4. Ezra DG, Beaconsfield M, Collin R. Surgical anatomy of the upper eyelid: old controversies, new concepts. Expert Rev Ophthalmol. 2009;4(1):47-57. 5. Pauly M, Sruthi R. (2019). Ptosis: Evaluation and management. Kerala J Ophthalmol. 2019;31(1);11-16. 6. Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003;27(3):193-204. 7. Iliff JW, Pacheco EM. Duane’s Clinical Ophthalmology. (Tasman W, Jaeger E, ed.). Lippincott Williams and Wilkins; 2001. 8. Latting MW, Huggins AB, Marx DP, Giacometti JN. Clinical evaluation of blepharoptosis: distinguishing age-related ptosis from masquerade conditions. Semin Plast Surg. 2017;31(1):5-16. 9. Putterman AM. Margin reflex distance (MRD) 1, 2, and 3: Ophthal Plast Reconstr Surg. 2012;28(4):308-11. 10. Yadegari S. Approach to a patient with blepharoptosis. Neurol Sci. 2016;37(10):1589-96. 11. Lim J, Hou J, Singa R, et al. Relative incidence of blepharoptosis subtypes in an oculoplastics practice at a tertiary care center. Orbit. 2013;32(4):231-4. 12. Burkat C, Oh D, Yen M. Margin to reflex distance 1,2,3. Eyewiki. eyewiki.aao.org/Margin_to_reflex_distance_1,2,3. September 16, 2019. Accessed March 13, 2020. 13. Savar A, Blaydon S, Nakra T, Shore J. Ptosis surgery: In: Tasman W, Jaeger E eds. Duane’s Ophthalmology. Pennsylvania: Lippincott Williams & Wilkins; 2013. 14. Jacobs SM, Tyring AJ, Amadi AJ. Traumatic ptosis: evaluation of etiology, management and prognosis. J Ophthalmic Vis Res. 2018;13(4):447-52. 15. Bhatti MT, Eisenschenk S, Roper SN, Guy JR. Superior divisional third cranial nerve paresis: clinical and anatomical observations of 2 unique cases. Arch Neurol. 2006;63(5):771-6. 16. Kung N, Stavern GPV. Isolated ocular motor nerve palsies. Semin Neurol. 2015;35(05):539-48. 17. Martin TJ. Horner syndrome: a clinical review. ACS Chem Neurosci. 2018;9(2):177-186. 18. Beebe J, Kardon R, Thurtell M. The yield of diagnostic imaging in patients with isolated Horner syndrome. Neurol Clin. 2017;35(1):145-51. 19. McGrogan A, Sneddon S, Vries CS de. The incidence of myasthenia gravis: a systematic literature review. Neuroepidemiology. 2010;34(3):171-83. 20. Kupersmith MJ. Ocular myasthenia gravis: treatment successes and failures in patients with long-term follow-up. J Neurol. 2009;256(8):1314-20. 21. Gilbert ME, Savino PJ. Ocular myasthenia gravis. Int Ophthalmol Clin. 2007;47(4):93-103. 22. Antonio-Santos AA, Eggenberger ER. Medical treatment options for ocular myasthenia gravis. Curr Opin Ophthalmol. 2008;19(6):468-478. 23. Porter NC, Salter BC. Ocular myasthenia gravis. Curr Treat Options Neurol. 2005;7(1):79-88. 24. Gilbert M, De Sousa E, Savino P. Ocular myasthenia gravis treatment: the case against prednisone therapy and thymectomy. Arch Neurol. Dec 2007 (64):1790-2. 25. Kumar V, Kaminski HJ. Treatment of Myasthenia Gravis. Curr Neurol Neurosci Rep. 2011;11(1):89-96. 26. Silvestri N, Wolfe G. Myasthenia Gravis. Semin Neurol. 2012;32(03):215-226. 27. Song A, Carter K, Nerad J, et al. Steroid-induced ptosis: case studies and histopathologic analysis. Eye. 2008;22(4):491-5. 28. Silkis R, Lee H, Gills Ray V. Highly Active Antiretroviral Therapy–Associated Ptosis in Patients With Human Immunodeficiency Virus. Arch Ophthalmol. 2009;127(3):345-6. 27. Kubis KC, Danesh-Meyer HV, Savino PJ, Sergott RC. The ice test versus the rest test in myasthenia gravis. Ophthalmol. 2000;107(11):1995-8. 28. Ellis F, Hoyt C, Ellis F, et al. Extraocular muscle responses to orbital cooling (ice test) for ocular myasthenia gravis diagnosis. J AAPOS. 2000;4(5):271-81. 29. Nguyen C, Campbell C. Myotonic dystrophy type 1. CMAJ Can Med Assoc J. 2016;188(14):1033. 30. Thornton CA. Myotonic dystrophy. Neurol Clin. 2014;32(3):705-19. 31. de Castro FAA, Cruz AAV, Sobreira CF da R. Brow Motility in Mitochondrial Myopathy: Ophthal Plast Reconstr Surg. 2010;26(6):416-9. 32. Daut P, Steinemann T, Westfall C. Chronic exposure keratopathy complicating surgical correction of ptosis in patients with chronic progressive external ophthalmoplegia. Am J Ophthalmol. 2000;130(4):519-21. 33. Murchison AP, Bilyk JR, Savino PJ. Ptosis in neurologic disease. In:Black E, Nesi F, Calvano C, et al, eds. Smith and Nesi’s Ophthalmic Plastic and Reconstructive Surgery. New York, NY: Springer; 2012:361-92. |

