 |  |
Retinal vein occlusion (RVO) is second only to diabetic retinopathy as a leading cause of retinal vascular disease. We typically encounter a case of RVO every day in our retina practice, and patients often present with a history of hypertension or glaucoma. Although macular ischemia and neovascularization are complications that contribute to the deterioration of vision, the most common cause of vision loss is macular edema. It is important to treat the macular edema promptly, given the fact that both clinical trials and clinical practice show a penalty in delaying treatment.
Intravitreal pharmacotherapy has expanded the treatment options for macular edema associated with vascular retinopathies such as RVO. Such a treatment protocol can provide substantial visual and structural improvements.
Because macular edema is mediated by vascular endothelial growth factor (VEGF), treatment with anti-VEGF therapies such as Lucentis (ranibizumab, Genentech), Eylea (aflibercept, Regeneron) and Avastin (bevacizumab, Genentech) is particularly effective. However, RVO is a multifactorial disease and inflammation also plays a critical role in its pathogenesis. Hypoxia promotes inflammatory mediators, which increase permeability and lead to retinal-blood barrier breakdown, resulting in macular edema. To combat this inflammatory process, clinicians should consider intravitreal steroid implants, such as Ozurdex (Allergan), which inhibit vascular permeability and suppress inflammatory mediators. Treatment of macular edema related to RVO with Ozurdex can lead to a significant anatomical and functional improvement. The biodegradable implant delivers a high concentration of dexamethasone into the vitreous over a period of time. We have often observed effects of its release for an average of four months, and lower concentrations may be observed for up to six months, based on clinical trial data.1,2 This may be an option for treatment when continuously monthly injections are not suitable.
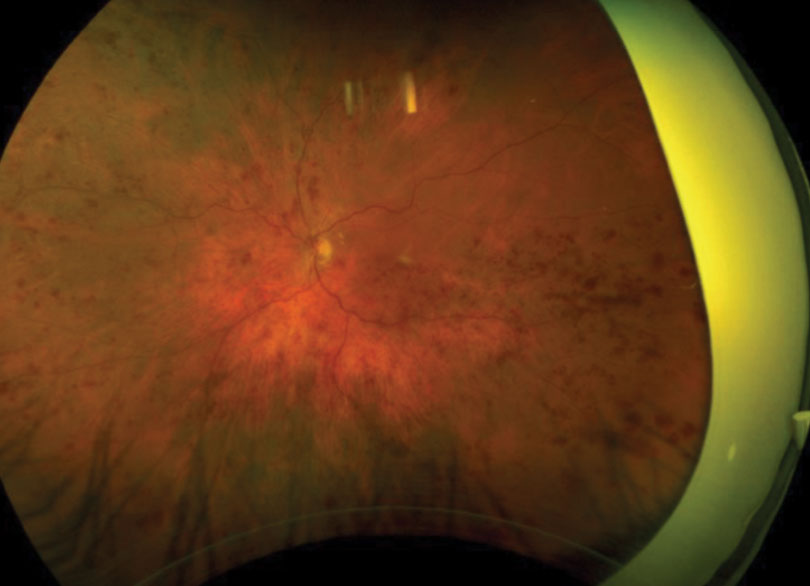 |
| Fig. 1. This patient presented with retinal hemorrhages and cotton-wool spots in all four quadrants of his left eye. Click image to enlarge. |
Ozurdex, FDA approved for macular edema related to RVO in 2009 following the GENEVA study, has since become a useful therapy in our office for selective cases of macular edema associated with RVO.2 Although the implant provides positive anatomical outcomes associated with decreased central retinal thickness (correlating with visual improvement), it comes with some adverse effects. These include cataract formation and increased intraocular pressure (IOP). Clinical trials show that up to 20% of patients will exhibit an IOP rise.1,2 In our experience, any IOP increase is usually transient and peaks by four to six weeks. Most cases of increased IOP in our practice have subsided once the steroid wears off and have been effectively managed with anti-glaucoma topical medications. However, clinicians must monitor the optic nerve, especially considering glaucoma may have been a contributing factor to the patient’s RVO.
When determining the best treatment options, clinicians must weigh the efficacy of the drug against the burden of re-injections and any potential associated complications.
One and Done
Case by Dr. Shechtman
A 62-year-old Caucasian male was referred for an evaluation of suspected central retinal vein occlusion (CRVO) OS. His medical history included hypertension for 15 years. His best-corrected visual acuity measured 20/25 OD and 20/40 OS. The dilated fundus examination was unremarkable for the right eye. The left eye revealed a healthy nerve with a cup-to-disc ratio of 0.4/0.4, dilated tortuous veins, retinal hemorrhages and a few cotton-wool spots in all four quadrants (Figure 1). Spectral-domain optical coherence tomography (SD-OCT) was consistent for the presence of macular edema OS (Figure 2). We confirmed the suspected diagnosis of CRVO with associated macular edema OS and initiated treatment with Lucentis.
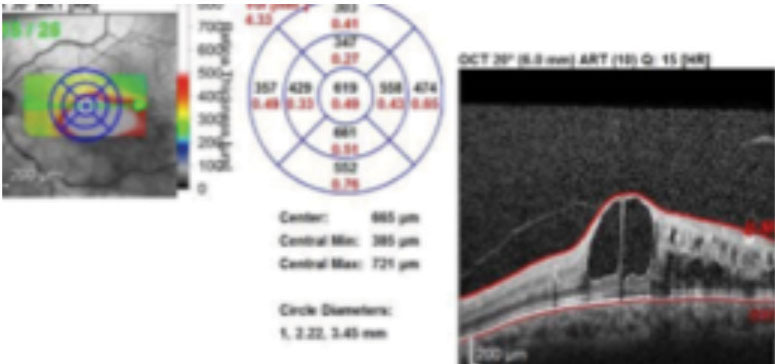 |
| Fig. 2. SD-OCT imaging confirmed macular edema in the left eye. Click image to enlarge. |
At the one-month follow up, his macular edema had decreased and his visual acuity improved to 20/30. The patient had another injection of Lucentis and was scheduled for another follow up in a month. At the next follow-up the macular edema had increased with deteriorating vision to 20/50. We then decided to try Ozurdex. One month after injecting the implant, the patient experienced a dramatic improvement of macular edema with restoration of normal macular contour (Figure 3). This correlated with a visual acuity gain to 20/25. The patient remained stable over a two-month period without the need for another injection. He had no noticeable increase of IOP during follow up.
Careful Monitoring
Commentary by Dr. Haynie
The treatment of macular edema secondary to a CRVO is crucial, considering chronic macular edema increases the risk of permanent visual loss, and the natural history of a CRVO is quite poor without intervention. When treating macular edema secondary to a CRVO in my practice, the retina surgeons will typically initiate treatment with Avastin, Lucentis or Eylea. If the patient fails to respond (presenting with recurrent or persistent edema) to an induction treatment of three injections, the next consideration is the use of steroids, whether it’s Triesence (triamcinolone acetonide injectable suspension, Novartis), Kenalog (triamcinolone acetonide injectable suspension, Bristol-Myers Squibb) or Ozurdex.
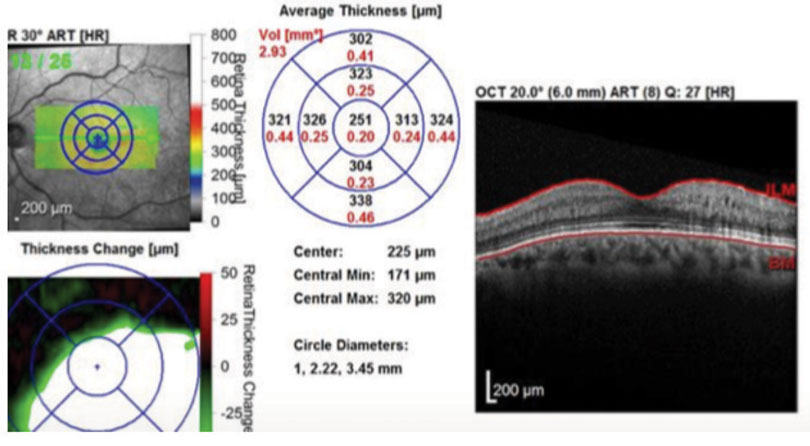 |
| Fig. 3. One month after implanting Ozurdex, the patient’s edema was once again under control with a normal macular contour. Click image to enlarge. |
As Dr. Shechtman noted, clinicians must weigh the risks and benefits of intravitreal steroids, given the risk of elevated IOP. In addition, Ozurdex is not FDA approved for phakic patients without a planned cataract surgery with future intraocular lens placement.
Intravitreal steroids were found to be effective for the treatment of macular edema secondary to a RVO in SCORE 1 and SCORE 2 clinical trials and since have become great adjuncts to anti-VEGF compounds in cases of chronic or recurrent macular edema.3,4 The risk of elevated IOP following intravitreal steroids can be as high as 20%, warranting close follow up with IOP monitoring—monthly based on our practice protocol. If we are concerned about a patient’s steroid response, we consider a trial of topical Durezol (Novartis) QID. If they experience no increase in IOP within four weeks, the surgeons feel comfortable using intravitreal steroids.
In the event of elevated IOP, the patient should be managed with topical anti-hypertensive agents for at least six months from the date of the intravitreal steroid treatment. In these cases clinicians should avoid prostaglandin analogs, as they can contribute to inflammatory ocular disease and cause cystoid macular edema (both present in patient with RVOs). Provided the patient has no systemic contraindications to carbonic anhydrase inhibitors, I tend to start with dorzolamide or Azopt (Novartis) TID, as this class of pharmaceutical can lower IOP and also reduce macular edema.
Patients with macular edema secondary to a CRVO should be referred for treatment based on clinical trial data that show a visual benefit from treatment that exceeds the natural history of the disease, which can lead to marked loss of visual function.
Dr. Girschek will be one of the primary retina specialists at Retina Macula Specialists of Miami, starting July 2019.
|
1. National Horizon Scanning Centre (NHSC). Dexamethasone intravitreal implant (Posurdex) for macular oedema secondary to central or branch retinal vein occlusion. Horizon Scanning Technology Briefing. Birmingham, UK: National Horizon Scanning Centre (NHSC); 2009. 2. Haller JA, Bandello F, Belfort R Jr, et al; Ozurdex Geneva Study Group. Randomized, sham-controlled trial of dexamethasone intravitreal implant in Ozurdex (dexamethasone intravitreal implant) patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(6):1134-46. 3. Scott IU, Vanveldhuisen PC, Oden NL, et al. Baseline characteristics and response to treatment of participants with hemiretinal compared with branch retinal or central retinal vein occlusion in the standard care vs corticosteroid for retinal vein occlusion (SCORE) study: SCORE study report 14. Arch Ophthalmol. 2012;130(12):1517-24. 4. Scott IU, VanVeldhuisen PC, Ip MS, et al. Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: the SCORE2 randomized clinical trial. JAMA. 2017;317(20):2072-87. |

