 |
We all know the highly vascularized choroid supplies blood to nourish the outer retina with oxygen and nutrients.1,2 But it also serves other purposes, and its dysfunction is the source of several pathological conditions we often come across in practice.2 Be especially vigilant for these three.
1. Vascular Abnormalities
The choroid comprises the most posterior portion of the uvea. It is predominantly made up of blood vessels and, to a lesser degree, fibroblasts, melanocytes and supporting connective tissue. In fact, the choroid is the most highly vascularized structure in the entire body, given the high rate of blood flow necessary to nourish the retina.2 This is made possible through the presence of choriocapillaris, a highly anastomosed network of fenestrated blood vessels, which is thickest at the fovea and thins peripherally.
The blood vessels are classified into two layers: Haller’s layer, which lies adjacent to Bruch’s membrane and contains large diameter vessels, and Sattler’s inner layer, which houses medium and small vessels that feed the capillary network and lies closer to the scleral surface.1,2
Due to this abundance of blood supply and flow, the choroid is the most common site for metastatic disease, accounting for over 90% of uveal metastasis.3 Cancers originating in the breast (47%), lung (21%) or gastrointestinal tract (4%) are the most common to metastasize by hematogenous spread, so highly vascularized areas such as the choroid are most likely to be affected.4
Other vascular abnormalities may arise in the choroid as well, such as bleeding or the formation of choroidal neovascular membranes. For example, poly-poidal choroidal vasculopathy (PCV) is an idiopathic condition where bleeding occurs in the peripapillary subretinal area (Figure 1).5
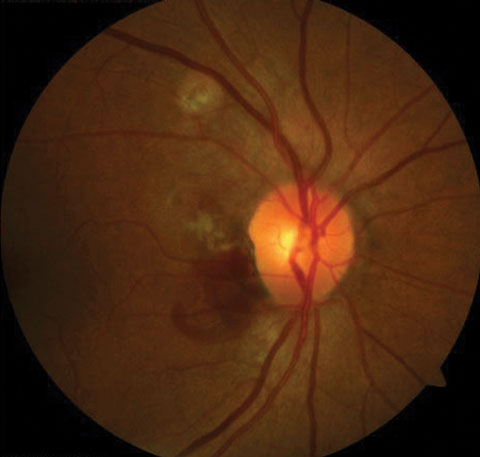 |
| Fig. 1. PCV signifies subretinal bleeding. Click image to enlarge. |
2. Lymphatic Dysfunction
In addition to the choriocapillaris, the choroid contains multiple lacunae. In animal studies, these lacunae have been identified as a site of lymphatic drainage.2 The presence of lymphatics in the human choroid is controversial and not yet well established, though most recent evidence points to the existence of nontraditional lymphatics.6 The lymphatic system generally functions to preserve fluid homeostasis and plays a key role in immunosurveillance.6,7 In vertebrates it may also be implicated in tumor metastasis, further contributing to choroidal metastasis.7
An imbalance of tissue fluid homeostasis may alter permeability as well as choroidal thickness.2 Increased choroidal permeability is also implicated in central serous chorioretinopathy, which manifests as a serous retinal detachment with or without the presence of pigment epithelial defects.8
Choroidal thinning has been associated with many pathological conditions, such as age-related macular degeneration, high myopia and ocular growth.9 Additionally, since lymphatics are known to play a fundamental role in immunosurveillance, the choroid is susceptible to uveitis and other choroidal inflammatory conditions.6
3. Melanoma
Unlike in animals, the human choroid represents the site of large and abundant melanocytes, which give the choroid its dark pigmentation and appearance. These melanocytes are situated in close proximity to the choroidal blood vessels.2 Besides pigmentation and light absorption, the function of melanocytes is unknown. However, their presence allows for cancer to arise in the form of a primary malignant choroidal melanoma.10
1. Summers JA. The choroid as a sclera growth regulator. Exp Eye Res. 2013(9);114:120-7. 2. Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144-68. 3. Arepalli S, Kaliki S, Shields CL. Choroidal metastases: Origin, features, and therapy. Indian J Ophthalmol. 2015;63(2):122-7. 4. Cohen VML. Ocular metastases. Eye. 2013;27:137-41. 5. Honda S, Matsumiya W, Negi K. Polypoidal choroidal vasculopathy: clinical features and genetic predisposition. Ophthalmologica. 2014;231:59-74. 6. Alexander JS, Becker F. Evidence for nontraditional lymphatics in the choroid. Invest Ophthalmol Vis Sci. 2015;56(2):1328. 7. Koina ME, Baxter L, Adamson SJ, et al. Evidence for lymphatics in the adult developing human choroid. Retinal Cell Biology. 2015;56(2):1310-27. 8. Kim HC, Cho WB, Chung H. Morphologic changes in acute central serous chorioretinopathy using spectral domain optical coherence tomography. Korean J Opthalmol. 2012;26(5):347-54. 9. Adhi M, Ferrara D, Mullins RF, et al. Characterization of choroidal layers in normal aging eyes using en face swept source optical coherence tomography. PLOS One. 2015:1-13. 10. Coupland SE, Lake SL, Zeschnigk M, et al. Molecular pathology of uveal melanoma. Eye (Lond). 2013;27(2):230-42. |

