 |
A 69-year-old Caucasian female presented for evaluation of blurred vision in both eyes for the past six months. She also noted increased light sensitivity. Her last eye exam was two years prior.
Her medical history was significant for Type 2 diabetes mellitus for 10 years and hypertension. She was currently taking Januvia (sitagliptin, Merck) and a combination medication glipizide and metformin HCl for blood sugar control and Catapres (Clondine, Boehringer Ingelheim Promeco) and Norvasc (amlodipine besylate, Pfizer) for control of her blood pressure (BP).
She was hospitalized six months earlier for extremely elevated hypertension. At that time, her BP was 250/119. She claims to have much better control now.
Evaluation
On examination, her best-corrected acuity measured 20/40 in each eye. Confrontation visual fields were full-to-careful finger counting OU. Her ocular motility testing was normal and her pupils were equally round and reactive without an afferent papillary defect. Her anterior segment was significant for 2+ nuclear sclerosis in both eyes. Tensions by applanation measured 18mm Hg OU.
On dilated fundus exam, her optic nerves appeared heathy with a small cup and good rim coloration and perfusion OU. Obvious retinal changes were seen in both eyes and are available for review (Figure 1). There was no neovascularization. Spectral-domain optical coherence tomography (SD-OCT) and OCT-Angiography (OCT-A) were performed and is also available for review (Figure 2).
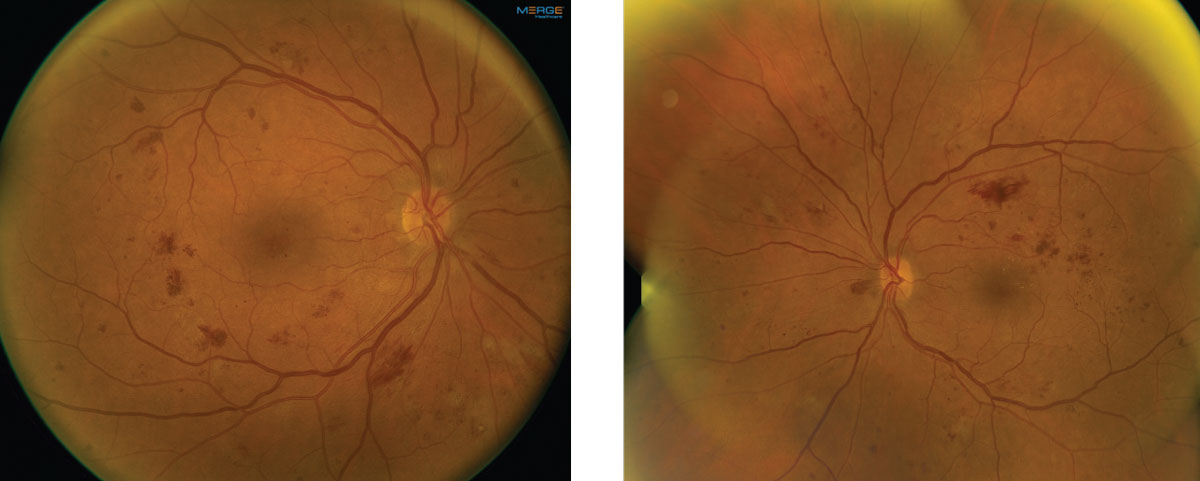 |
| Fig. 1. These fundus images show the right and left eye of our patient. Note the extent of involvement. Click image to enlarge. |
Take the Retina Quiz
1. How would you categorize the retinopathy in both eyes?
a. Stage 4 hypertensive retinopathy.
b. Moderate non-proliferative diabetic retinopathy.
c. Severe non-proliferative diabetic retinopathy.
d. Proliferative diabetic retinopathy.
2. What are the essential findings on the spectral domain optical coherence tomography and optical coherence tomography angiography in both eyes?
a. Center involved diabetic macular edema.
b. Non-center involved diabetic macular edema.
c. Widening of the foveal avascular zone and no macular edema.
d. Both A and C are correct.
3. How do you account for the reduced acuity in each eye?
a. Nuclear sclerosis cataract.
b. Diabetic macular edema.
c. Ischemia.
d. Combination of A and C.
4. Which of the following would not be an indication for treating this patient?
a. Moderate non-proliferative diabetic retinopathy.
b. Any level of diabetic retinopathy if center-involved diabetic macular edema is present.
c. Severe non-proliferative diabetic retinopathy.
d. Proliferative diabetic retinopathy.
5. How should this patient be managed?
a. Close observation.
b. Intravitreal anti-vascular endothelial growth factor injections.
c. Panretinal photocoagulation.
d. Both A and B.
Diagnosis
Significant retinal hemorrhages are noticeable in all four quadrants in both our patient’s eyes, as are scattered cotton wool spots. On careful examination, no neovascularization can be seen in either eye. Based on the early treatment diabetic retinopathy study (ETDRS) classification, these findings meet the criteria for severe non-proliferative diabetic retinopathy (NPDR).
The diagnosis of severe NPDR is based on the 4:2:1 rule of the ETDRS classification:
- 4: Significant retinal hemorrhages in all 4 quadrants,
- 2: Venous beading in 2 or more quadrants
- 1: Intraretinal microvascular abnormalities in at least 1 quadrant.
Any one of these findings meet the criteria for severe NPDR.
Discussion
Once a patient progresses to severe NPDR, their risk of progressing to proliferative diabetic retinopathy (PDR) in one year increases more than 50%, compared with mild or moderate NPDR where the risk of PDR in one year is 5% and 12% respectively.1 Because of this increased risk for progressing to PDR, patients with severe NPDR should be seen every three to four months compared with mild or moderate NPDR in which the follow up is one year.
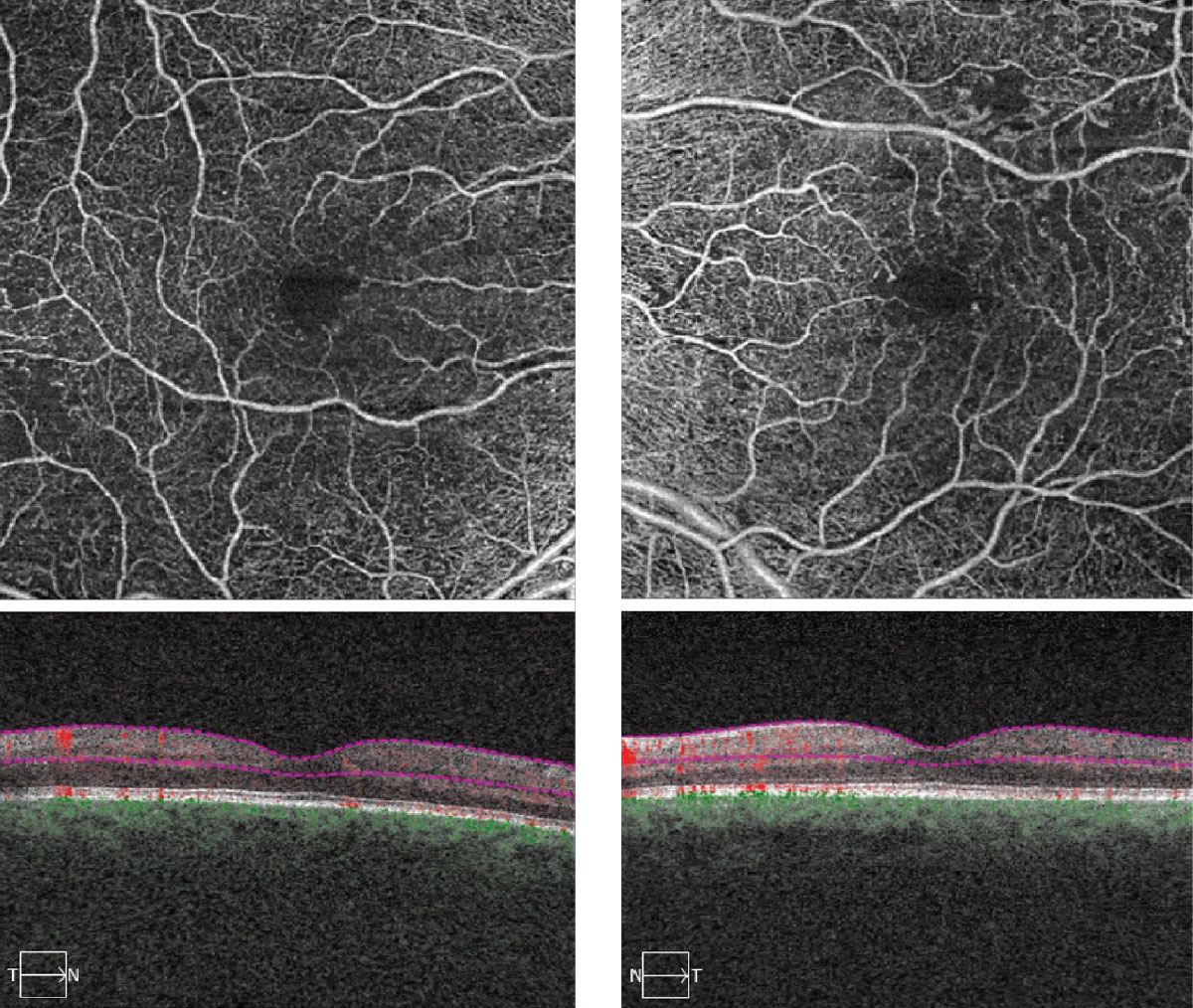 |
| Fig. 2. These OCT-A images represent 6x6mm of the right and left eyes. Note the changes within each macula. Click image to enlarge. |
The indications for treatment of DR has evolved in recent years. Traditionally, treatment was recommend for patients with clinically significant macular edema (CSME) or if there is PDR. In the era of OCT and treating DME with intravitreal anti-vascular endothelial growth factor (VEGF) injections, the treatment for DME has evolved. Instead of determining if macular edema is clinical significant, now retinal specialists want to know if the DME is center involved (CI-DME) or non-center involved based on OCT imaging. CI-DME is generally treated with an anti-VEGF injection whereas non CI-DME may be observed. The anti-VEGF drugs have supplanted laser for the treatment of DME and is now considered the standard for the treatment of DME.
The treatment of choice for PDR is less certain. The DRCR.net established the efficacy of ranibizumb in the Protocol S study, which compared traditional panretinal photocoagulation (PRP) with intravitreal ranibizumab.2 Both drugs were efficacious however, the patients with ranibizumab had slightly better outcomes. Specifically, the ranibizumab patients had better visual field preservation and were less likely to need pars plana vitrectomy over a five-year period. Visual acuity outcomes were similar in both groups. Despite these outcomes, most retinal specialists still use PRP as a first line therapy or in combination with the anti-VEGF medications. The preference of PRP may be due to the burden of treatment that is required with the anti-VEGF drugs, as well as cost.
The Research
What about treating patients earlier, before they go on to develop PDR? This was the question posed in a post hoc analysis of 756 patients that were part of the RIDE and RISE clinical trials.3
The purpose of their retrospective study was to examine DR outcomes after two years in patients who at baseline had severe NPDR (and DME). In this study diabetic retinopathy patients were categorized based on the Diabetic Retinopathy Severity Scale (DRSS), which ranges from 10 to 75. Patients with moderately severe to severe NPDR were categorized as levels 47 to 53. What the authors discovered was that 78% of the eyes with moderately severe and severe NPDR treated with ranibizumab saw a ≥2-step regression in the severity of their DR compared with only 12% that were treated in the sham group.3
The author conducted a similar study looking at Aflibercept in patients with level 47 to 53 retinopathy on the DRSS. However, this was a prospective study of 402 patients that were randomized to receive a sham injection vs. aflibercept given either every eight weeks or every 16 weeks (after three initial monthly doses). Their outcomes were quite similar to what was seen with ranibizumab. At one year, up to 80% of patients treated with aflibercept had a >2-step regression in the severity of their DR compared with only 15% in the sham group.4
The results of both of these studies suggest that patients with severe NPDR would benefit from anti-VEGF therapy instead of waiting until patients develop PDR. What’s more, with earlier treatment we can actually see a significant regression in diabetic retinopathy. Unfortunately most retinal specialists have been very slow to adopt this changing paradigm, again, likely due to the burden of patients needing multiple treatments as well as the costs and risks associated with anti-VEGF treatments. It’s also important to realize that most all of these patients have presumably normal vision given they don’t have any DME.
Getting back to our patient, we did refer her to a retinal specialist for consideration of treatment. We felt her vision was reduced due to a combination of cataracts and some ischemia in the fovea. She did not have DME but on OCT-A we could see some widening and irregularity of the foveal avascular zone in both eyes, more so in the left eye due to ischemia.
|
1. Chan S, Bubela T, Dimopoulos I, et al. Choroideremia research: report and perspectives on the second international scientific symposium for choroideremia. Ophthalmic Genet. 2016 Sep;37(3):267-75. 2. Freund P, Sergeev Y, MacDonald I. Analysis of a large choroideremia dataset does not suggest a preference for inclusion of certain genotypes in future trials of gene therapy. Molecular Genetics & Genomic Medicine. 2016;4(3):344-58. 3. Imani S, Ijaz I, Shasaltaneh M, et al. Molecular genetics characterization and homology modeling of the CHM gene mutation: a study on its association with chorioderemia. Mutation Research. 2018;775(2):39-50. 4. MacDonald I, Hume S, Chan S, et al. Chorioderemia. GeneReviews. February 26, 2015. www.ncbi.nlm.nih.gov/books/NBK1337/. Accessed June 6, 2018. |
Retina Quiz Answers:
1) c; 2) c; 3) d; 4) a; 5) d.

