Age-related macular degeneration (AMD), a bilateral, progressive retinal disease, is a major health problem that is too often undiagnosed. Although most optometrists think they have more glaucoma patients in their practice than they do AMD patients, AMD is three times as prevalent as glaucoma. In fact, AMD is more common than all of glaucoma and diabetic retinopathy combined.1-3
A recent study found approximately 25% of eyes rated as normal during a primary eye care provider’s dilated eye examination had macular characteristics indicative of AMD, revealed by fundus photography and trained raters.4 The reasons for the missed diagnoses remain unclear, but improved AMD detection strategies are needed to ensure patients receive appropriate treatment before irreversible vision loss occurs.
Traditionally, AMD is diagnosed when a dilated fundus examination reveals clinically evident drusen or RPE changes. A litany of diagnostic testing for AMD exists, including optical coherence tomography (OCT), OCT angiography (OCT-A), multispectral imaging, fundus autofluorescence and photography, microperimetry, electroretinography and retinal angiography.
But optometrists can truly stay ahead of AMD if they change the way they approach the disease, by identifying AMD risk during routine examinations and by using a new diagnostic tool, dark adaptation, which can identify the earliest functional biomarker of AMD. This method of identifying “AMD suspects” is much like diagnosing glaucoma suspects—a careful history and clinical examination may reveal risk factors that warrant further testing to determine if the disease is truly present.
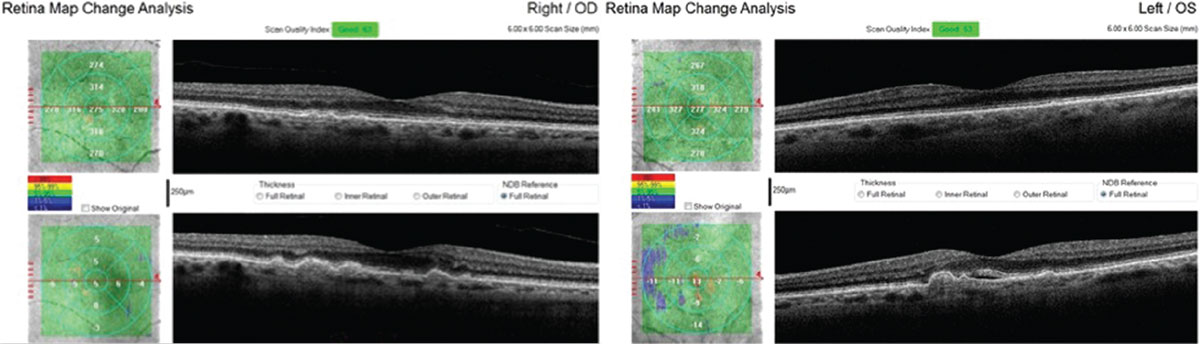 |
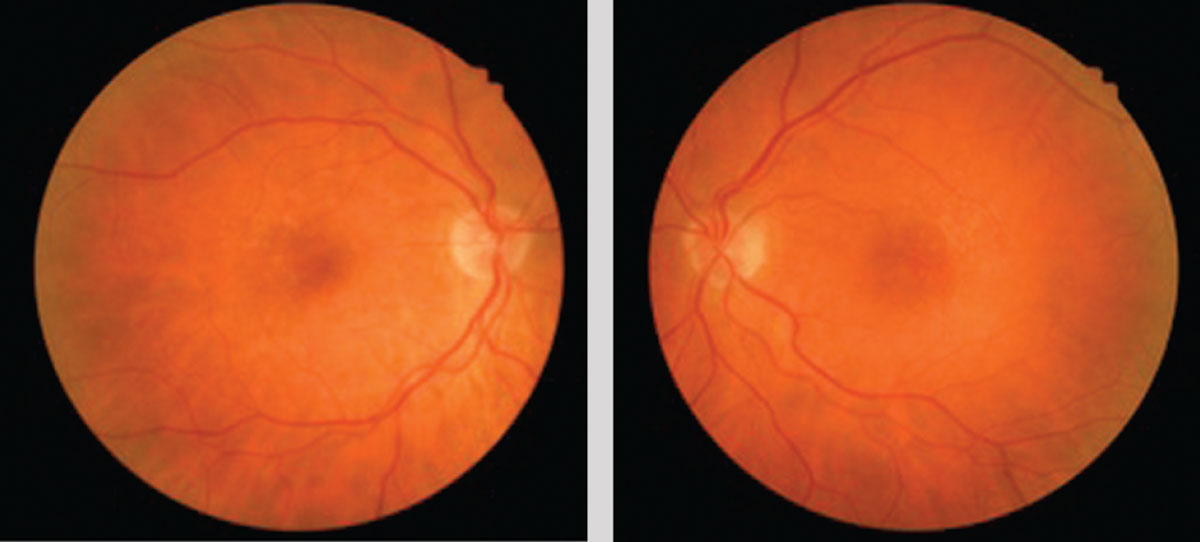
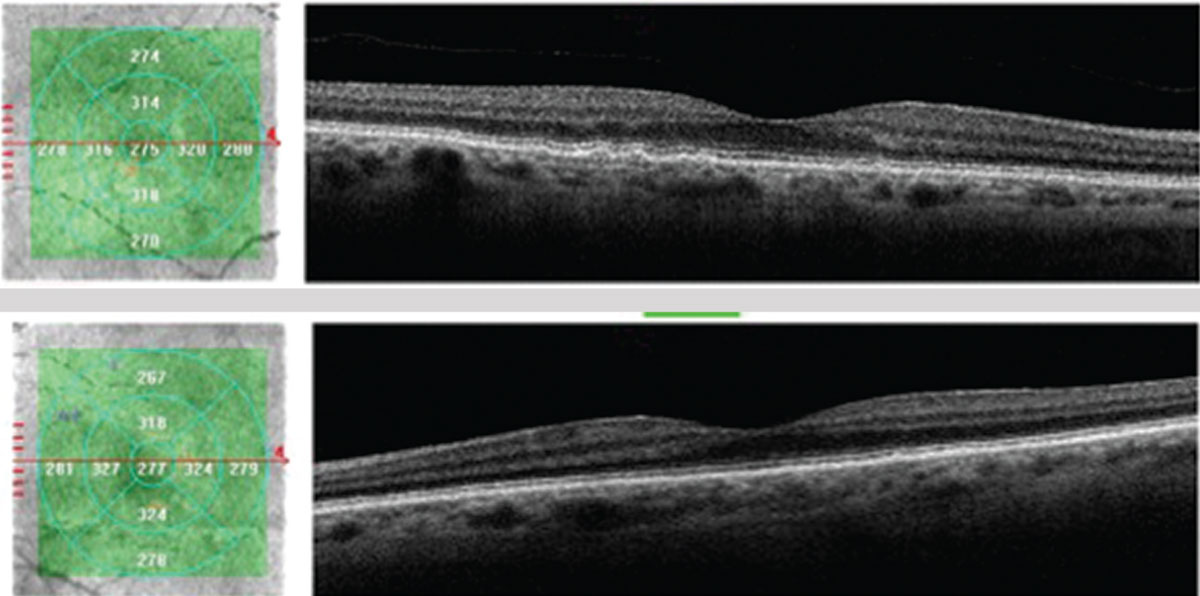
| This 71-year-old Caucasian female with small, hard drusen OU had abnormal dark adaptation and was diagnosed with AMD in 2016 (top OCT images). Even with treatment, the size and number of her drusen worsened and her dark adaptation progressed over time. She was being monitored routinely with dilated examinations every three months due to this quick progression and high risk of converting to wet AMD. On one of these routine exams, her visual acuity was 20/25-1 OD and 20/25+2 OS. She had no symptoms to report, and her Amsler grid had been stable during both at-home and in-office testing. Dilated exam revealed macular thickening OS, which was confirmed as subretinal fluid on OCT. The patient was referred to a retinologist who confirmed the presence of CNV with fluorescein angiography and initiated anti-VEGF treatment the same week. Today, her visual acuity remains 20/25 OS and she is still asymptomatic (bottom OCT images). Click image to enlarge. |
A New Look at AMD
The macula encompasses the most posterior region of the retina within the vascular arcades and, as a whole, has significantly more rods than cones—only the central fovea is cone dominated in the macula.5 Rod loss is the beginning of AMD, as rods are affected earlier and more severely than cones throughout the disease progression. Only much later in the disease process are cones affected, which correlates with a decrease in visual acuity.6 Because rod deterioration happens in the earliest stages of AMD, dark adaptation becomes affected much earlier than visual acuity declines.7
Research shows AMD causes anatomic and functional consequences before clinical signs of drusen appear. However, by the time drusen are visible, the patient has already had AMD for several years. Drusen are a manifestation of cholesterol locally produced by the retinal pigment epithelium (RPE) and deposited as a wash under the entire macula in Bruch’s membrane. This microscopic cholesterol layer is the hallmark defect of AMD, as electron microscopy in donor eyes has proven patients without AMD do not have this abnormal layer of cholesterol. Although the layer is not yet visible in living eyes using current diagnostic tools, it does significantly delay dark adaptation, even early in the disease before clinical signs are evident.7,8
As the disease progresses, this cholesterol layer continues to accumulate, resulting in focal areas sufficiently thickened to be clinically visible as drusen. This additive cholesterol accumulation causes inflammation, oxidative stress and disruption of oxygen and nutrients (such as vitamin A, a deficiency of which further hinders dark adaptation) supplied to the outer retina. Ultimately, this causes organized apoptosis of photoreceptors in the macula.9,10 The retina naturally combats oxidative stress with its own antioxidants: the human macula pigments consisting of lutein and zeaxanthin.11 In addition, antioxidant nutritional supplementation can help patients delay vision loss in AMD.
Macular pigment optical density (MPOD) measures the macular density of these pigments to determine if patients have an adequate amount to combat this stress.
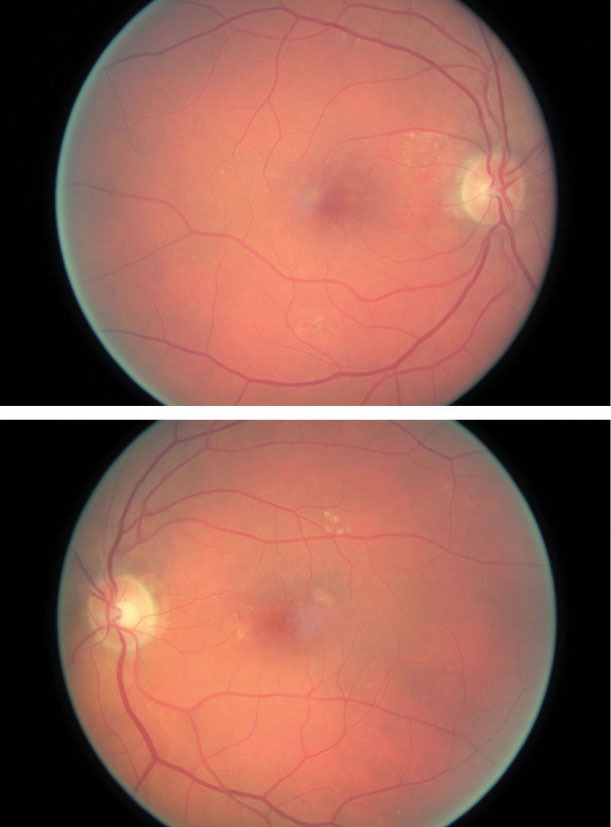 |
| The same patient’s color photography shows drusen with mild RPE changes in both eyes with mild macular thickening beginning in the left eye (bottom) that coincides with subretinal fluid identified with OCT. Click image to enlarge. |
Identifying AMD Risk
Risk factors for AMD identifiable with careful history include age, family history, Caucasian race, smoking, obesity and comorbid conditions such as hypertension, hypercholesterolemia and diabetes.12-14 While declining night vision and contrast sensitivity occur in normal aging, these symptoms occur more severely and more quickly in macular degeneration; therefore, it is reasonable to consider these symptoms as potential risk factors when diagnosing AMD.4 During clinical examination, any evident drusen, RPE mottling or both can be considered structural risk factors for AMD, similar to how increased or asymmetric optic nerve cupping causes glaucoma suspicion.15 As with variable cup-to-disc ratios in glaucoma, drusen and RPE changes can be normal age-related changes or early signs of true macular degeneration—risk factors, not diagnostic signs. Patients with drusen 125µm or larger, indistinct soft or reticular drusen, total drusen area of half the disc or more and hyperpigmentation are statistically at higher risk for AMD.15
Newer testing can now identify additional risk factors such as reduced macular pigment density. MPOD instruments measure the ratio of central blue light absorption to the peripheral absorption rate to determine the amount of macular pigment present. The more blue light absorbed centrally, the higher the MPOD score, which correlates to denser macular pigment.16 Research shows higher MPOD scores are associated with a slower AMD progression rate and lower risk of advancing to severe AMD. Low MPOD levels are associated with increased risk of progression to late AMD.17
The Age Related Eye Disease Study 2 (AREDS2) found that adding lutein and zeaxanthin to the original AREDS 1 formulation and removing the beta carotene reduced the risk of progression to advanced AMD compared with those taking the original AREDS 1 formulation.18 This effect was even more pronounced in patients who had low intake of dietary lutein and zeaxanthin. However, participants in AREDS2 participants had significantly higher lutein and zeaxanthin serum levels compared with the general population. But even these well-nourished participants had significantly reduced risk of progression with lutein and zeaxanthin supplementation than those without.18
Thus, although low MPOD is not diagnostic of AMD, it is a strong risk factor for developing AMD and the likelihood of disease progression. Moreover, MPOD is a modifiable risk factor because supplementation can increase low MPOD scores and decrease risk of progression to severe AMD.4,19,20
Determining genetic risk is somewhat complex; at least 19 genes are known to be involved in various AMD pathways, including the complement, immune/inflammatory, extracellular matrix and DNA repair/protein binding pathways.21,22 A growing body of literature suggests a subset of genetic variables along with demographic, behavioral and ocular signs can be highly predictive of risk to advanced AMD and vision loss.22 Individuals at a higher genetic risk should be considered for earlier screening or, if diagnosed with the disease, should be monitored closer as they are at a higher risk of progression.
New Technology for Diagnosis and Management
Evaluating structure and function together is extremely helpful in clinical practice to help diagnose AMD and possibly determine the rate and extent of progression. Studies of structure to diagnose AMD and its associated findings including include OCT, OCT-A, color photography, multispectral imaging, fundus autofluorescence and fluorescein angiography. Studies of function include hyperacuity perimetry, microperimetry, Amsler grid and visual acuity. As clinicians, enhanced confidence in detailed macular evaluation and proper interpretation of test findings aids in earlier diagnosis of AMD and differentiating it from other macular diagnoses. Here is a look at several new tools available to help clinicians diagnose AMD as early as possible and manage patients throughout the treatment regimen:
Dark adaptation. This is the only functional biomarker of AMD and does not measure risk.23-27 Although dark adaptation does have a tendency to decline with normal aging process, it is statistically more severe in AMD. It can be measured using the AdaptDx adaptometer (Maculogix), which uses a bleaching flash and then records the response to stimuli until the rod intercept (the time it takes for the eye to adjust from bright light to darkness) is reached. The test experience is similar to a visual field and takes from a few moments up to 20 minutes. The AdaptDx has normative values to differentiate between normal age-related night vision decline and true abnormalities in the rod intercept time found in early AMD. For the detection of AMD, the test has a high sensitivity, correctly identifying 90.6% of confirmed AMD cases as well as a high specificity, identifying 90.5% of confirmed normal cases.7,23
DA DifferentialsConditions other than AMD that can present with poor dark adaptation include genetic retinal disorders and vitamin A deficiency. In the aging population at risk for AMD, these differential conditions will present with retinal findings of inherited retinal disease or will have a systemic history of malnutrition. Thus, they are fairly simple to rule out with patient history, clinical examination and ancillary testing.23-27 |
Fundus autofluorescence (FAF). Because the RPE plays a significant role in the development and advancement of AMD, understanding its metabolic health is extremely helpful. General observations of FAF patterns in AMD suggest that elevated lipofuscin causes increased FAF in early stages of AMD associated with drusen and RPE metabolic stress, while decreasing RPE lipofuscin is associated with advancing stages of AMD as the RPE is damaged and ultimately stops lipofuscin production.28,29
FAF is particularly helpful in identifying basal laminar drusen, which signal a higher risk of advancement to wet AMD.30 Basal laminar drusen present on FAF as drusenoid lesions that significantly outnumber clinically evident drusen or drusen visible with other modalities.31 Identifying basal laminar drusen earlier in disease process allows earlier intervention and close monitoring for the development of choroidal neovascularization (CNV).
Studies show patterns of FAF in both CNV and geographic atrophy (GA).32,33 On FAF, classic CNV presents as focal darkening in an area of confirmed retinal thickening––likely due to the position of CNV and blockage of natural autofluorescence. An increase in autofluorescence may be noted inferior to the precise location of leakage, illustrating a gravitational pooling of lipofuscin. However, these findings are inconsistent for recent-onset, small-area occult CNV or retinal angiomatous proliferative lesions.32
FAF is more accurate in delineating the area and progression of GA, as it reveals a markedly decreased (black) signal with sharp borders. In some cases, a border of hyperautofluorescence is seen around the GA lesion—a strong indicator of likely GA progression.33 The exact borders of GA are more easily identified by FAF than by color funduscopy, yielding a more accurate assessment of the extent and anticipated progression.
OCT and OCT-A. The cross-sectional, semi-histological images obtained with OCT have become invaluable in AMD management. Not only does OCT report qualitative data but also quantitative measurements of increased thickness due to drusen or fluid or decreasing atrophic changes in micrometers with serial imaging. OCT can localize and detect RPE detachments, subretinal fluid, intraretinal fluid, RPE tears, disciform scars and new neovascular membranes.34
OCT-A is a new and promising diagnostic tool for the detection of CNV, although it is still considered a noninvasive complement to current testing such as fluorescein angiography. Currently, OCT-A does not image slow leakage well, as it can only detect blood flow above a minimum threshold.35 However, future research will improve these capabilities to hopefully decrease the need for more invasive testing.35
Macular microperimetry and hyperacuity perimetry. This is a test of macular function that correlates sensitivity abnormalities with a precise fundus location based on the visual skill of hyperacuity. By correlating function with structure, early metamorphopsia functional change can be identified and confirmed with other structural imaging techniques. In intermediate AMD, microperimetry can detect localized functional changes of atrophy or neovascularization.36 By comparing these functional changes to a specific retinal locale with OCT or FAF, clinicians can identify the precise size and location of advancing disease. Microperimetry can also track progression over time by determining what changes are statistically significant and not an inherent variation.37
The ForeseeHome automated, at-home device is a tool that transmits monthly hyperacuity testing reports to the prescribing doctor to review. In addition, if a statistically significant change is noted the doctor is immediately alerted to perform a dilated fundus examination sooner than the routinely scheduled visit. Research shows 94% of patients using the ForeseeHome device who converted to wet AMD retained better than 20/40 vision compared with only 62% of patients using conventional methods.38
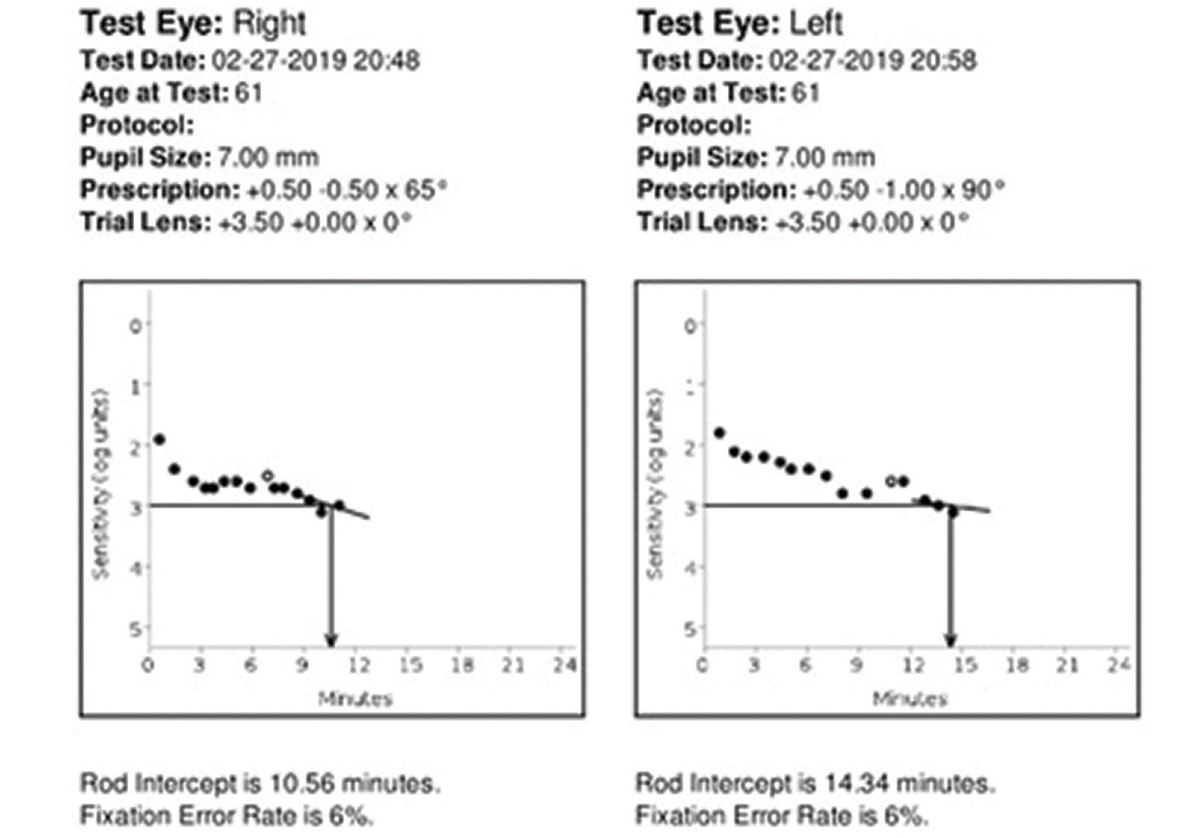
Case ExampleA 61-year-old Caucasian female presented with 20/20 best-corrected vision and a chief complaint of deteriorating night vision, particularly when driving. She denied a family history of AMD. She is not a smoker, is not overweight and has no systemic diagnoses or medications. Because she is older than 50, Caucasian and had a complaint of night vision decline, we recommended she undergo dark adaptation testing due to concern of moderate AMD risk. Dilated fundus exam also revealed small, deep, symmetrical drusen in both eyes that was subtle but present. Her dark adaptation was markedly abnormal at 10.56 minutes OD and 14.34 minutes OS. A normal rod intercept time is considered less than 6.5 minutes. After correlating these findings, the patient was diagnosed with early dry AMD in both eyes. She was given extensive education about the condition and the importance of regular follow-up care to monitor for progression. We recommended she take supplements, use UV-blocking sunglasses outdoors and blue-blocking lenses indoors, focus on a healthy lifestyle and diet and remain compliant with routine primary care visits to evaluate and manage any future comorbid conditions. She is also now monitored every six months with dilated fundus examination for progression to possible wet AMD.
|
Management
With no cure for AMD, the goal is to delay or prevent the onset of late-stage AMD with early diagnosis and therapy. In addition, early diagnosis of conversion to wet AMD allows early intervention with anti-vascular endothelial growth factor (VEGF) therapy, which shows improved long-term visual prognosis. However, despite long-term anti-VEGF therapy, patients still have a risk of AMD progression, itself resulting in vision loss.39,40
By using risk factor analysis, new technologies such as dark adaptation and careful dilated exam, AMD can be diagnosed earlier than ever before. Patient education and management recommendations include lifestyle changes such as weight control, exercise and healthy diet as well as control of comorbid conditions such as diabetes, hypertension and high cholesterol—all of which can improve long-term outcomes. Studies show UV protection outdoors and blue-blocking lens protection indoors can help to reduce the risk of progression of AMD in studies.41-44 A serious recommendation of smoking cessation is essential, as this is the foremost modifiable risk factor that contributes to the development of advanced AMD.45
Nutritional supplementation has become a controversial topic in recent years, but overall research recommends prescribing a high quality carotenoid supplement over no supplementation.46 Because nutraceuticals are not FDA regulated, it is important that clinicians recommended a specific brand at the doctor’s discretion.
To promptly identify conversion to wet AMD, clinicians must properly educate patients on the difference between dry and wet AMD and the importance of watchful waiting with routine dilated examinations. Patients should be monitored in two to six month intervals, depending on the individual patient and their risk factors and the rate of progression over time. Follow-up should consist of a careful dilated macular examination as well as testing.
Serial dark adaptation testing can help clinicians assess how quickly the patient’s disease state is progressing and make an informed decision about how often the patient should be monitored. The dark adaptation rod intercept score can change significantly over time, and monitoring it provides greater confidence when determining monitoring schedules.
Detecting conversion to wet AMD as soon as possible is crucial to avoid rapid vision loss. Therefore, to truly stay ahead of AMD, clinicians must diagnose the disease in the earliest stages possible, initiate treatment and provide patient education—all of which will hopefully prevent advancement to late-stage disease.
Dr. Legge is in private practice in Wyomissing, PA, and has an advanced studies certification in retinal disease from Salus University. Disclosure: Dr. Legge is a speaker for Maculogix.
|
1. Klein R, Chou CF, Klein BE, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129(1):75-80. 2. Kempen JH, O’Colmain BJ, Leske MC, et al; Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122(4):552-63. 3. National Eye Institute. Open-angle glaucoma defined. https://nei.nih.gov/eyedata/glaucoma. Accessed April 18, 2019. 4. Neely DC, Bray KJ, Huisingh CE, et al. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol. 2017;135(6):570-5. 5. Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497-523. 6. Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37(7):1236-49. 7. Owsley C, McGwin G, Clark M, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016;123(2):344-51. 8. Pikuleva IA, Curcio CA. Cholesterol in the retina: the best is yet to come. Prog Retin Eye Res. 2014;41:64-89. 9. Owsley C, McGwin G, Jackson GR, et al. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47(4):1310-18. 10. Hecht S, Mandelbaum J. The relation between vitamin A and dark adaptation. JAMA Ophthalmol. 1939;112(19):1910-16. 11. Beatty S, Murray IJ, Henson DB, et al. Macular pigment and risk for age-related macular degeneration in subjects from a northern European population. Invest Ophthalmol Vis Sci. 2001Feb;42(2):439-46. 12. Chakarvarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010 Dec;10:31. 13. Flamendorf J, Agrón E, Wong WT, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015;122(10):2053-62. 14. Kleiner R, Enger C, Alexander MF, Fine SL. Contrast sensitivity in age-related macular degeneration. Arch Ophthalmol. 1988;106(1):55-57. 15. Wang JJ, Foran S, Smith W, Mitchell P. Risk of age-related macular degeneration in eyes with macular drusen or hyperpigmentation. The Blue Mountains Eye Study Cohort. Arch Ophthalmol. 2003;121(5):658-63. 16. Sharpe LT, Stockman A, Knau H, Jgle H. Macular pigment densities derived from central and peripheral spectral sensitivity differences. Vision Res. 1998;38(21):3233-9. 17. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry. 2004;75(4):216-30. 18. Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005-15. 19. Hammond BR, Johnson EJ, Russell RM, et al. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci. 1996;38(9):1795-1801. 20. Weigert G, Kaya S, Pemp B, et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(11):8174-78. 21. Seddon JM, Rosner, B. Validated prediction models for macular degeneration progression and predictors of visual acuity loss identify high-risk individuals. Am J Ophthalmol. 2019 Feb;198:223-61. 22. Ratnapriya R, Chew EY. Age-related macular degeneration—clinical review and genetics update. Clin Genet. 2013;84(2):160-66. 23. Jackson GR, Scott IU, Kim IK, et al. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(3):1427-31. 24. Yang GQ, Chen T, Tao Y, Zhang ZM. Recent advances in the dark adaptation investigations. Int J Ophthalmol. 2015;8(6):1245-52. 25. Iannaccone A. Measuring dark adaptation in the elderly: a predictor of who may develop macular degeneration? Invest Ophthalmol Vis Sci. 2014;55(8):4790. 26. Owsley C, Huisingh C, Jackson GR, et al. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Invest Ophthalmol Vis Sci. 2014;55(8):4776-89. 27. Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002;1(3):381-96. 28. Solbach U, Keilhauer C, Knabben H, Wolf S. Imaging of retinal autofluorescence in patients with age-related macular degeneration. Retina 1997;17(5):385-9. 29. Delori F, Fleckner MR, Goger DG, et al. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41(2):496-504. 30. Curcio C, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Opthalmol. 1999;117(3):329-39. 31. Meyerle C, Smith RT, Barbazetto IA, Yannuzzi LA. Autofluorescence of basal laminar drusen. Retina. 2007;27(8):1101-6. 32. Rufino S, Bandello F, Cunha-Vaz JG. Fundus autofluorescence in exudative age-related macular degeneration. Br J Ophthalmol. 2007;91(4):491-6. 33. Holz FG, Bindewald-Wittich A, Fleckenstein M, et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143(3):463-72. 34. Hee M, Baumal CR, Puliafito CA, et al. Optical coherence tomography of age-related macular degeneration and choroidal neovascularization. Ophthalmology. 1996;103(8):1260-70. 35. Rodriguez F, Staurenghi G, Gale R. The role of OCT-A in retinal disease management. Graefes Arch Clin Exp Ophthalmol. 2018;256(11):2019-26. 36. Midena E, Radin PP, Pilotto E, et al. Fixation pattern and macular sensitivity in eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. A microperimetry study. Sem in Ophthalmol. 2004;19(1-2):55-61. 37. Midena E, Vujosevic S, Convento E, et al. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91(11):1499-503. 38. Chew EY, Clemons TE, Bressler SB, et al; AREDS2-HOME Study Research Group. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121(2):535-44. 39. Zarubina A, Gal-Or O, Huisingh C, Owsley C. Macular atrophy development and subretinal drusenoid deposits in vascular endothelial growth factor treated age-related macular degeneration. Invest Ophthal Vis Sci. 2017;58(14):6038-45. 40. Danis RP, Lavine JA, Domalpally A. Geographic atrophy in patients with advanced dry age-related macular degeneration: current challenges and future prospects. Clin Ophthalmol. 2015 Nov;9:2159-74. 41. Sui G-Y, Liu G-C, Liu G-Y, et al. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br J Ophthalmol. 2013;97(4):389-94. 42. Pipis A, Touliou E, Pillunat LE, Augustin AJ. Effect of the blue filter intraocular lens on the progression of geographic atrophy. Eur J Ophthalmol. 2015;25(2):128-33. 43. Glazer-Hockstein C, Dunaief J. Could blue light–blocking lenses decrease the risk of age-related macular degeneration? Retina. 2006;26(1):1-4. 44. Taylor HR, Muñoz B, West S, et al. Visible light and risk of age-related macular degeneration. Trans Am Ophthalmol Soc. 1990;88:163-78. 45. Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108(4):697-704. 46. Carneiro A, Andrade JP. Nutritional and lifestyle interventions for age-related macular degeneration: a review. Oxid Med Cell Longev. 2017;2017: 6469138. |