 |
Q: I may start fitting scleral lenses for irregular corneas and even presbyopia. How can I best optimize vision with this modality in this patient population?
A: “Scleral lenses are a great specialty lens option for a multitude of patients,” says Nicholas Gidosh, OD, chief of the Cornea and Contact Lens Service at the Pennsylvania College of Optometry. “With a host of indications, including irregular corneas, ocular surface disease and even high ametropia, they can be customized to work for many of our patients.” Dr. Gidosh notes that an exciting area of improvement with these lenses is in presbyopic correction.
 |
|
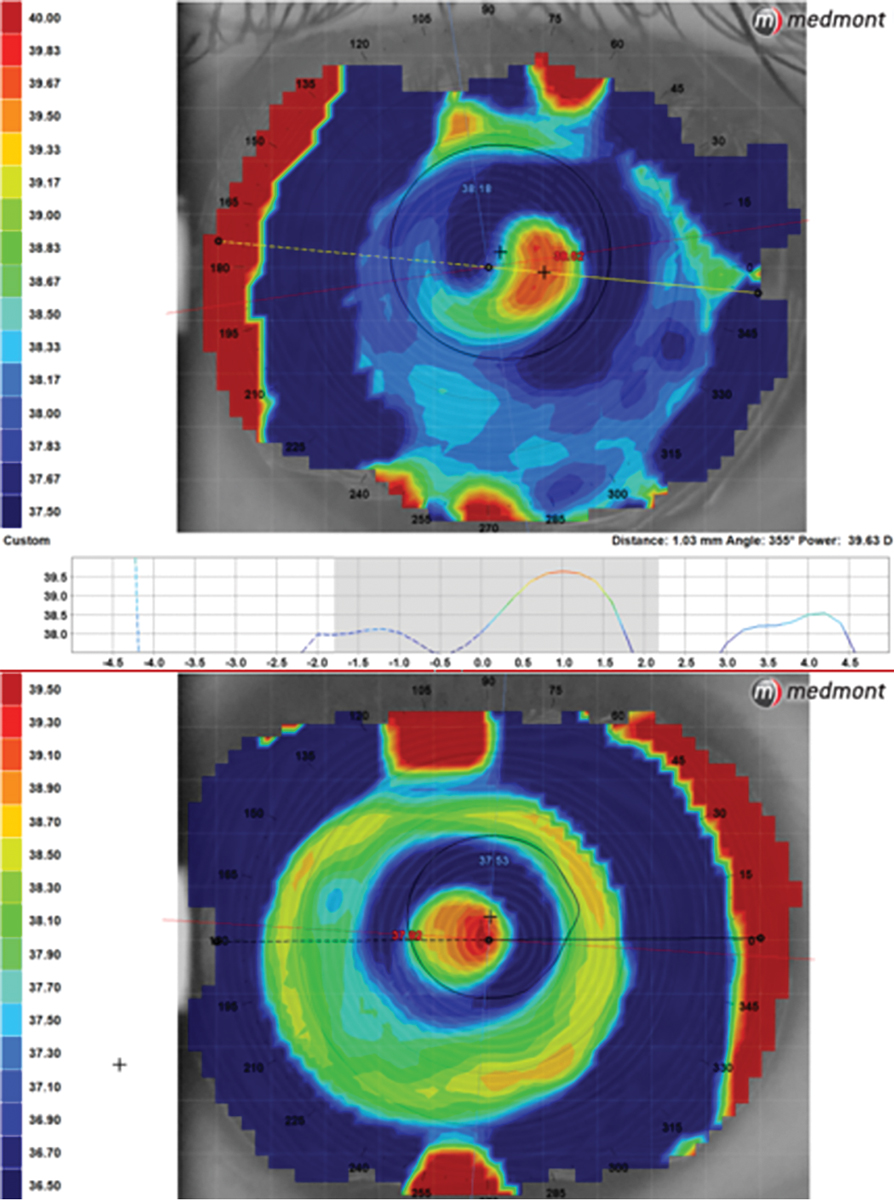
Topography measuring 1mm of temporal lens decentration in a center-near lens (top map) contrasted with a scleral multifocal using decentered optics to align the near zone with the visual axis (bottom). Click image to enlarge. |
Troubleshooting Techniques
Scleral lenses fit stably on most eyes, so the multifocal designs often rely on simultaneous multifocal optics, according to Dr. Gidosh. This can be achieved through center distance or near aspherics, or sometimes through concentric designs. Currently, the most popular method used to achieve multifocal correction in scleral lenses is through center near aspherics, he notes. The issue with these optical designs is that scleral lenses tend to decenter in the inferior-temporal direction. With high amounts of decentration, patients may struggle to obtain adequate near vision. Luckily, there are several techniques that can be used to troubleshoot this issue.
The first involves improving lens centration and increasing near zone size, says Dr. Gidosh. Ways to improve scleral lens centration include optimizing clearance over the surface of the eye, selecting the most appropriate lens diameter and obtaining a more aligned edge using toric or asymmetric peripheral curves. Another modification involves increasing the size of the near zone, which puts more of the add power into the pupil to allow better vision at near without adversely affecting distance vision.
The second technique is to use a scleral design that incorporates a standardized amount of optical zone decentration, Dr. Gidosh notes. Specialty labs are aware of the issue of lens decentration, and some have determined the average amount typically seen. Knowing this, the labs can include a predetermined amount of near zone decentration to better align the add zone within the patient’s visual axis. The zone size can still be modified to further refine the vision to meet the patient’s needs.
Dr. Gidosh adds that the final technique is by diagnostic measurement of near zone decentration. This can be done by using a trial lens set with markings to measure the amount and direction of lens decentration. Knowing this value, the lab can then recenter the near zone to align it with the visual axis. An alternative to this is beginning with a standard center near trial lens and measuring the amount of the zone’s decentration using topography. These methods help the labs precisely determine the amount and axis of near zone decentration needed for best visual results.
Final Considerations
While near vision can sometimes be difficult to achieve with scleral multifocal lenses, there are several troubleshooting methods to improve visual function. Patients may also benefit from enlarged near zones in their nondominant eye to enhance near performance or a smaller near zone in the dominant eye to aid with distance tasks like driving. Some designs also allow for center distance optics to further improve distance in the dominant eye. Understanding these customizations and when to use them will help providers achieve better results with this modality, Dr. Gidosh says.
Dr. Shovlin, a senior optometrist at Northeastern Eye Institute in Scranton, PA, is a fellow and past president of the American Academy of Optometry and a clinical editor of Review of Optometry and Review of Cornea & Contact Lenses. He consults for Kala, Aerie, AbbVie, Novartis, Hubble and Bausch + Lomb and is on the medical advisory panel for Lentechs.
1. Deol M, Taylor DA, Radcliffe NM. Corneal hysteresis and its relevance to glaucoma. Curr Opin Ophthalmol. 2015;26(2):96-102. 2. Ortiz D, Piñero D, Shabayek MH, et al. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. J Cataract Refract Surg. 2007;33(8):1371-5. 3. Radcliffe NM, Tracer N, De Moraes CGV, et al. Relationship between optic disc hemorrhage and corneal hysteresis. Can J Ophthalmol. 2020;55(3):239-44. 4. Congdon NG, Broman AT, Bandeen-Roche K, et al. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006;141(5):868-75. 5. Kamiya K, Miyata K, Tokunaga T, et al. Structural analysis of the cornea using scanning-slit corneal topography in eyes undergoing excimer laser refractive surgery. Cornea. 2004;23(8 Suppl):S59-64. 6. Jaycock PD, Lobo L, Ibrahim J, et al. Interferometric technique to measure biomechanical changes in the cornea induced by refractive surgery. J Cataract Refract Surg. 2005;31(1):175-84. 7. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714-20. 8. Abitbol O, Bouden J, Doan S, et al. Corneal hysteresis measured with the Ocular Response Analyzer in normal and glaucomatous eyes. Acta Ophthalmol. 2010;88(1):116-9. 9. Agarwal DR, Ehrlich JR, Shimmyo M, et al. The relationship between corneal hysteresis and the magnitude of intraocular pressure reduction with topical prostaglandin therapy. Br J Ophthalmol. 2012;96(2):254-7. 10. Tracer N, Ayoub S, Radcliffe NM. The association between corneal hysteresis and surgical outcomes from trabecular meshwork microinvasive glaucoma surgery. Graefes Arch Clin Exp Ophthalmol. 2021;259(2):475-81. 11. Anand A, De Moraes CGV, Teng CC, et al. Corneal hysteresis and visual field asymmetry in open angle glaucoma. Invest Ophthalmol Vis Sci. 2010;51(12):6514-8. 12. Medeiros FA, Meira-Freitas D, Lisboa R, et al. Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study. Ophthalmology. 2013;120(8):1533-40. 13. Sharifipour F, Panahi-Bazaz M, Bidar R, et al. Age-related variations in corneal biomechanical properties. J Curr Ophthalmol. 2016;28(3):117-22. 14. Zhang C, Tatham AJ, Abe RY, et al. Corneal hysteresis and progressive retinal nerve fiber layer loss in glaucoma. Am J Ophthalmol. 2016;166:29-36. 15. Cohen EJ. Keratoconus and normal-tension glaucoma: a study of the possible association with abnormal biomechanical properties as measured by corneal hysteresis. Trans Am Ophthalmol Soc. 2009;107:282-99. 16. Wong BJ, Moghimi S, Zangwill LM, et al. Relationship of corneal hysteresis and anterior lamina cribrosa displacement in glaucoma. Am J Ophthalmol. 2020;212:134-43. |

