There are substantially more mechanisms and connections in play within dry eye disease (DED) than just a lack of tears. As defined in 2007 by the Dry Eye WorkShop, DED is a multifactorial disease afflicting millions of individuals worldwide.1-6 Various intrinsic and extrinsic risk factors, to name just a few, include gender, genetics, tear quality, age, hormones, immune status, nutrition, pathogens, contact lenses/refractive surgery and environmental stress, any of which can alter the harmonious balance within the ocular surface.
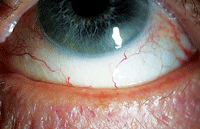 | |
| Meibomian gland dysfunction is notoriously intertwined with dry eye disease. Indeed, the majority of dry eye patients are likely to have an underlying meibomian gland disorder. Photo: Kelly Nichols, OD, MPH, PHD |
The pathophysiology of this condition is far reaching—sometimes the outward ocular surface signs can pale in comparison to the chronic internalized damage. Subsequently, complications can increase the potential susceptibility to desiccation and epithelial injury. In turn, a vicious cycle develops in which inflammation amplifies and fosters further damage to the ocular surface by chronic deregulation of the lacrimal functional unit and tear composition.
In this article, both new and recognized clinical insights illustrate how even a small change from a concomitant disease state can have a big impact on ocular surface anatomy.
Identifying the Players
The lacrimal functional unit is a self-contained integrated system comprising the ocular surface (cornea, conjunctiva and meibomian glands) and the lids, which is linked directly to primary and accessory lacrimal glands with a feedback connective loop that includes both motor and sensory nerves.7-10 Furthermore, when DED exerts its influence on the lacrimal function unit, there is a chronic inflammatory echo, similar to a sound wave, that ripples through the ecosystem, creating an inherent breakdown in tear chemistry. The piercing or triggering effect causing the ocular surface destabilization can be connected to endogenous and microbial stress, antigen localization and epigenetic factors.11,12
Tear integrity issues—the chipping away of the epithelial/stromal corneal barrier—can be devastating to the lacrimal function unit. Consequently, exposure of these underlying basal corneal layers creates a vacuum-like effect that increases the risk of ulcerative infection and collagenous scar deposition. In addition, a vast degree of conjunctival goblet cell dropout reduces efficiency of mucin production and causes scarring and wrinkling that can lead to conditions such conjunctival chalasis.13
Finally, lid disease cannot be underestimated in its relation to tear quality.14,15 It has been established that, like glaucoma, there is an inflow (internal) and outflow (external) pathway in the meibomian gland anatomy. For the internal component, meibomian glands contain several acini for which secretory cells called meibocytes produce meibomian oils (meibum). For the external component, the meibum fluid is transported through a ductal system where it is shuttled to the anterior external lid margin surface by the contractile forces of the orbicularis and the muscle of Riolan.
 |
|
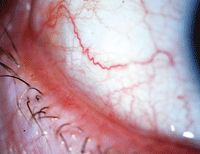
| With symptoms that are easily mistaken for dry eye disease, conjunctival chalasis is characterized by loose or redundant conjuntival tissue. Photo: John P. Herman, OD |
However exquisite this system might seem, the deficiency in the model can be traced back to androgens, blink reflex and, as more recent reports have shown, to Demodex mites. Consequently, the pathophysiological breakdown in the lid structure initiates signaling cascades of tumor necrosis factor-alpha and other pro-inflammatory mediators within these tissues manifesting in cytolysis and hyperkeratinization.14,16,17
Now that we understand the anatomy, let’s take a close look at three common disease states that are often concomitant with DED.
Consider Conjunctival Chalasis
Conjunctival chalasis (CChal) is a very common and frustrating ocular surface condition—a study in Japan found that it occurs in more than 98% of individuals over the age of 60 (1,388 patients).18
Although it is easily mistaken for DED, CChal’s chief distinction is that patients usually present with pain and discomfort at the chalasis site due to redundant conjunctiva (typically temporal) that becomes loose because of the absence of Tenon’s fascia.
Symptoms are associated with age greater than 50 and dry eye history, and it has a tendency to occur after cataract surgery, blepharoplasty or other surgical procedure that involved peribulbar or retrobulbar anesthesia.19 Also of interest, a prospective study found the prevalence of CChal in autoimmune thyroid disease to be as high as 88%.20
Even though pain and foreign body sensation are differentiating characteristics, conjunctival chalasis is linked to evaporative dry eye by the dynamic interference in the normal tear film. Clinically, it can be observed as a distinctly elevated tear meniscus centrally, with a broken up nasal and temporal tear film.21 In addition, check for tear clearance with fluorescein or lissamine green; these stains will quickly show “bunching” of the conjunctival tissue apposed to the inferior lid margin for which the lack of tear flow on the blink reflex is akin to the frictional forces associated with lid wiper epitheliolopathy.22-24
Inflammation may have a role in the pathogenesis of conjunctival chalasis. Significant amounts of selective pro-inflammatory markers—matrix metalloproteinase-9 (MMP-9), interleukin-1 beta, interleukin-6 and interleukin-8—have been found in the tear film of patients with CChal.25,26
A useful pearl when attempting to localize the chalasis site: Ask the patient to point to the affected area. Once identified, place your thumb on the external eyelid and apply gentle pressure to the site while the patient looks up and down. To properly assess the CChal severity, the technique must be done without anesthetic in the eye.
Treat CChal with palliative remedies such as artificial tears, short-term use of a corticosteroid (such as loteprednol) or with amniotic membrane transplantation.27-30
Demodex : A ‘Mitey’ Masquerader
Perhaps one of the most confounding conditions in your chair today, tomorrow or the next day will simply be a Demodex mite lid infestation. These particular mites are a significant culprit in many cases of blepharitis and can be concomitant with DED due to their chronic nature. Patients with an ocular Demodex infestation often complain of itching, burning, redness, crusting at the base of the lashes, blurry vision and dry eye.31,32 Additionally, some patients will anecdotally report they have more symptoms in the morning, especially after taking a hot shower.
Many of us overlook this small ectoparasite (class Arachnid, order Acarina) in search of a “better” differential diagnostic solution to why all of our conventional treatment pathways have failed.
To further complicate matters, there are two distinct species:33,34
• Demodex folliculorum has a comparatively long body and proliferates at the base of the lashes (cylindrical dandruff) causing anterior blepharitis. Specifically, Demodex folliculorum consume epithelial cells at the hair follicle causing lash distention, hypoplasia and reactive hyperkeratinization, which is commonly observed as trichiasis and madarosis.
 | |
|
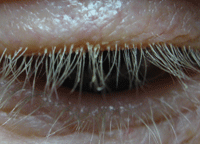
Cylindrical sleeves on the eyelash are characteristic of Demodex eyelid infestation.
Photo: Ron Melton, OD, and Randall Thomas, OD, MPH
|
• Demodex brevis, as its name implies, has shorter size and burrows deep into the sebaceous and meibomian glands, causing posterior blepharitis. Demodex brevis mechanically blocks meibomian gland orifices, leading to insufficient tear lipid secretion, which can be an inflammatory trigger for dry eye.
Interestingly, Demodex mites carry their own bacterial reservoirs that can contribute to ocular surface inflammation. The bacteria on the surface of the mite skin has been shown to compete with known staphylococcal species, producing a human host immune response leading to an inflammatory eyelid and periorbital epidermal to subepidermal reaction.35 If the disease is in a chronic and progressive phase, the inflammation will spread to the conjunctiva and cornea, potentially leading to infiltrative keratoconjunctivitis, nodular scar deposition and corneal neovascularization.
The good news is that there are now specific treatments available to mediate Demodex infestation. Recent to the ophthalmic marketplace is Cliradex (Bio-Tissue), a concentrated tea tree oil derivative based on the potency of the active ingredient 4-terpineol to kill Demodex mites.36 Other available treatments include BlephEx (Rysurg) and the Demodex Convenience Kit (Ocusoft), as well as compounded off-label oral and topical ivermectin, which is currently used in the veterinary community and now being studied in human subjects.37,38
Recurrent Corneal Erosion
Recurrent corneal erosion (RCE) is a painful and sometimes even incapacitating condition for many patients. The majority of cases occur secondary to a superficial traumatic injury, yet the pathophysiology of RCE is only partially understood.39
In the last several years, researchers have used confocal laser technologies to further investigate the etiology of recurrent erosions and were able to establish that there was altered epithelial cell types coupled with inflamed stromal keratocytes compared to those cells found within normal corneal physiology. The hypotheses from these studies indicate the origins of the trauma—whether from a glancing fingernail abrasion, edge piece of paper, leaf or branch, DED, previous herpetic keratitis, or other epithelial/stromal dystrophies—are rooted in a smoldering inflammatory cascade of MMP-9 and MMP-2. The expression of these particular enzymes causes an upregulation of pro-inflammatory mediators, leading to basement membrane degradation and poor epithelial membrane adhesion. Consequently, the process of re-epithelialization to recurrent sloughing off of these same cells could be directly related to the preexisting events.40-42
The link between RCE and DED is mechanical in nature. Logically, when the ocular surface is desiccated via a dry eye mechanism from contact lens wear, previous surgery, autoimmune disease or other anterior basement/stromal dystrophy condition, the dominos are set into motion priming the epithelium to dismount by the shearing force of the lids sliding over the weakened tissue. As a result, the patient tends to awaken in the middle of the night or morning with a neuropathic shockwave of corneal pain.43
From a clinical perspective, be sure to clearly communicate the visual expectations and prognosis. Furthermore, when managing recurrent corneal erosion, more than one treatment will be necessary, especially in severe cases. Fortunately, multiple modalities are available, such as oral doxycycline, autologous serum, topical loteprednol, cyclosporine, phototherapeutic keratectomy and amniotic corneal transplantation.
By discussing the various therapeutic options and surgical techniques, the optometrist can ensure a preventive strategy that will make the experience easier and more tolerable for the patient.44
|
|
|
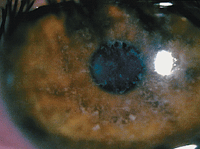
Deposits in the epithelial basement membrane, shown here, may be partly to blame for recurrent corneal erosion, which is sometimes mistaken for dry eye disease.
Photo: Joseph P. Shovlin, OD
|
For ocular surface disease management, today is the day to effect change. Take the opportunity to grasp that “carpe diem moment” and delve deeper into a patient’s history. You could make their day, or even change their life.
|
Pardon the Interruption: How Refractive Surgery Disrupts the Ocular Surface
Much of it has to do with the nerves. The cornea is one of the most densely innervated physiological structures of the body. A vast majority of these nerves branch off from the posterior corneal nerves and form three key networks throughout the cornea.1 One network is located in the mid-stroma, another in the anterior stroma and the third is located throughout the epithelium.1 The cornea also contains a high density of sympathetic nerve fibers that aid in epithelial metabolism, proliferation and wound healing.1 Given that refractive surgery of all types affects one or all of these networks, it’s no surprise that these patients can have postoperative chronic dry eye symptoms.2 Evidence of this dry eye phenomena can be observed at the one-week, one-month, three-month and six-month follow-up visits using tear break-up time, Schirmer’s test and corneal sensitivity as measures.3,4,8,9 Patients who have the most issues postoperatively tend to have a greater attempted refractive error correction, a greater ablation depth, are older and often are female.4,6,7 In addition, multiple retrospective studies have shown that patients with tear film instability before surgery are more likely to experience greater and more persistent symptoms postoperatively.2,5,7,8 One of the most significant measures of postoperative dry eye symptoms is a value less than 10mm from a preoperative Schirmer’s test.8 While DED is commonly linked to many other disease states—thyroid disease, Sjögren’s syndrome, hormonal changes, rheumatoid arthritis and diabetes—the fact that it’s also present in our refractive surgery patients cannot be dismissed. To truly serve our patients, we must take care to screen appropriately and treat our patients who suffer from dry eye preoperatively. By taking this extra proactive step, treatment outcomes will improve to ensure a more successful experience for our surgical patients.
1. Remington LA. Clinical Anatomy of the Visual System, 2nd ed. St. Louis: Elsevier/Butterworth-Heinemann; 2005.
|
Drs. Kabiri and Singh practice at Malik Eye Care, a multi-location MD/OD group setting in Queens, New York.
Dr. Cooper practices at an MD/OD group setting in Willimantic, Conn.
Dr. Burkart practices at a multi-location OD setting in Zionsville, Ind.
1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007 Apr;5(2):75-92.
2. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007 Apr;5(2):93-107.
3. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007 Apr;5:108-152.
4. Design and conduct of clinical trials: report of the Clinical Trials Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007 Apr;5(2):153-162.
5. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007 Apr;5(2):163-178.
6. Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007 Apr;5(2):179-93.
7. Beuerman RW, Mircheff A, Pflugfelder SC, Stern ME: The lacrimal functional unit. In: Pflugfelder SC, Beuerman RW, Stern ME, ed. Dry eye and ocular surface disorders, New York: Marcel Dekker; 2004.
8. Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998 Nov;17(6):584-9.
9. Stern ME, Beuerman RW, Fox RI, et al. A unified theory of the role of the ocular surface in dry eye. Adv Exp Med Biol. 1998;438:643-51.
10. Beuerman RW, Maitchouk DY, Varnell RJ, Pedroza-Schmidt L. Interactions between lacrimal function and the ocular surface. In: Kinoshita S, Ohashi Y, eds. Proceedings of the 2nd Annual Meeting of the Kyoto Cornea Club. The Hague, the Netherlands: Kugler Publications; 1998:1-10.
11. Zierhut, M, Stern ME, Sullivan DA. Immunology of the Lacrimal Gland, Tear Film and Ocular Surface. Abingdon, UK: Taylor and Francis; 2005.
12. McDermott AM, Perez V, Huang AJ. et al. Pathways of corneal and ocular surface inflammation: a perspective from the Cullen Symposium. Ocul Surf. 2005 Oct;3(4 Suppl):S131-8.
13. Behrens A, Doyle JJ, Stern L, et al; Dysfunctional tear syndrome study group. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006 Sep;25(8):900-7.
14. Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980 Mar;51(3):243-51.
15. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):1917-2064.
16. Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. Feb 2008;49(2):271-81.
17. Yano M, Kishida E, Iwasaki M. Docosahexaenoic acid and vitamin E can reduce human monocytic U937 cell apoptosis induced by tumor necrosis factor. J Nutri. May 2000;130(5):1095-1101.
18. Mimura T, Yamagami S, Usui T, et al. Changes of conjunctivochalasis with age in a hospital-based study. Am J Ophthalmol. 2009 Jan;147(1):171-7.
19. Hovanesian JA. Diagnosis and surgical treatment of conjunctival chalasis. Ocular Surgery News. May 5, 2009. Available at: www.healio.com/ophthalmology/blogs/hovanesian/diagnosis-and-surgical-treatment-of-conjunctival-chalasis. Accessed July 21, 2014.
20. de Almeida SF, de Sousa LB, Vieira LA, et al. Clinic-cytologic study of conjunctivochalasis and its relation to thyroid autoimmune diseases: prospective cohort study. Cornea. 2006 Aug;25(7):789-93.
21. Yeu E. Strategies for managing difficult-to-treat DED. Cataract & Refractive Surgery Today. 2013 March;13(3):44-5.
22. Korb DR, Greiner JV, Herman JP, et al. Lid-wiper epitheliopathy and dry-eye symptoms in contact lens wearers. CLAO J. 2002 Oct;28(4):211-6.
23. Korb DR, Herman JP, Greiner JV, et al. Lid wiper epitheliopathy and dry eye symptoms. Eye Contact Lens. 2005 Jan;31(1):2-8.
24. Korb DR, Herman JP, Finnemore VM, et al. An evaluation of the efficacy of fluorescein, rose bengal, lissamine green, and a new dye mixture for ocular surface staining. Eye Contact Lens. 2008 Jan;34(1):61-4.
25. Acera A, Rocha G, Vecino E, et al. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res. 2008 Oct;40(6):315-21.
26. Erdogan-Poyraz C, Mocan MC, Bozkurt B, et al. Elevated tear interleukin-6 and interleukin-8 levels in patients with conjunctivochalasis. Cornea. 2009 Feb;28(2):189-93.
27. Kruse FE, Meller D. Amniotic membrane transplantation for reconstruction of the ocular surface. Ophthalmologe. 2001 Sep;98(9):801-10.
28. Meller D, Tseng SC. Reconstruction of the conjunctival and corneal surface. Transplantation of amniotic membrane. Ophthalmologe. 1998 Dec;95(12):805-13.
29. Meller D, Tseng SC. Conjunctivochalasis: literature review and possible pathophysiology. Surv Ophthalmol. 1998 Nov-Dec;43(3):225-32.
30. Huang Y, Sheha H, Tseng SC. Conjunctivochalasis interferes with tear flow from fornix to tear meniscus. Ophthalmology. 2013 Aug;120(8):1681-7.
31. Basta-Juzbasic A, Subic JS, Ljubojevic S. Demodex folliculorum in development of dermatitis rosaceiformis steroidica and rosacea-related diseases. Clin Dermatol. 2002 Mar-Apr;20(2):135-40.
32. English FP,Nutting WB. Demodicosis of ophthalmic concern. Am J Ophthalmol. 1981 Mar;91(3):362-72.
33. Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089-3094. Invest Ophthalmol Vis Sci. 2005 Sep;46(9):3089-94.
34. Bevins CL, Liu FT. Rosacea: skin innate immunity gone awry? Nat Med. 2007 Aug;13(8):904-6.
35. Wolf R, Ophir J, Avigad J, et al. The hair follicle mites (Demodex spp.). Could they be vectors of pathogenic microorganisms? Acta Derm Venereol. 1988;68(6):535-7.
36. Tighe S, Gao YY, Tseng SC, et al. Terpinen-4-ol is the most active ingredient of tea tree oil to kill Demodex mites. Transl Vis Sci Technol. 2013 Nov;2(7):2.
37. Salem DA, El-Shazly A, Nabih N, et al. Evaluation of the efficacy of oral ivermectin in comparison with ivermectin-metronidazole combined therapy in the treatment of ocular and skin lesions of Demodex folliculorum. Int J Infect Dis. 2013;17(5):e343-7.
38. Schachter S. A different approach to treating Demodex blepharitis. Jan 3, 2014, Available at: http://optometrytimes.modernmedicine.com/optometrytimes/news/different-approach-treating-demodex-blepharitis. Accessed July 29, 2014.
39. Trobe JD, Laibson PR. Dystrophic changes in the anterior cornea. Arch Ophthalmol. 1972 Apr;87(4):378-82.
40. Labbe A, Nicola RD, Dupas B, et al. Epithelial basement membrane dystrophy: evaluation with the HRT II Rostock Cornea Module. Ophthalmology. 2006 Aug;113(8):1301-8.
41. Chikama T, Takahashi N, Wakuta M, et al. In vivo biopsy by laser confocal microscopy for evaluation of traumatic recurrent corneal erosion. Mol Vis. 2008;14:2333-9.
42. Sakimoto T, Shoji J, Yamada A, et al. Upregulation of matrix metalloproteinase in tear fluid of patients with recurrent corneal erosion. Jpn J Ophthalmol. 2007 Sep-Oct;51(5):343-6.
43. Mandell RB, Fatt I. Thinning of the human cornea on awakening. Nature. 1965;208:292.
44. Friedman NJ. Treating recurrent corneal erosion syndrome. Advanced Ocular Care. 2010 Jul/Aug;1(5):38-40.

