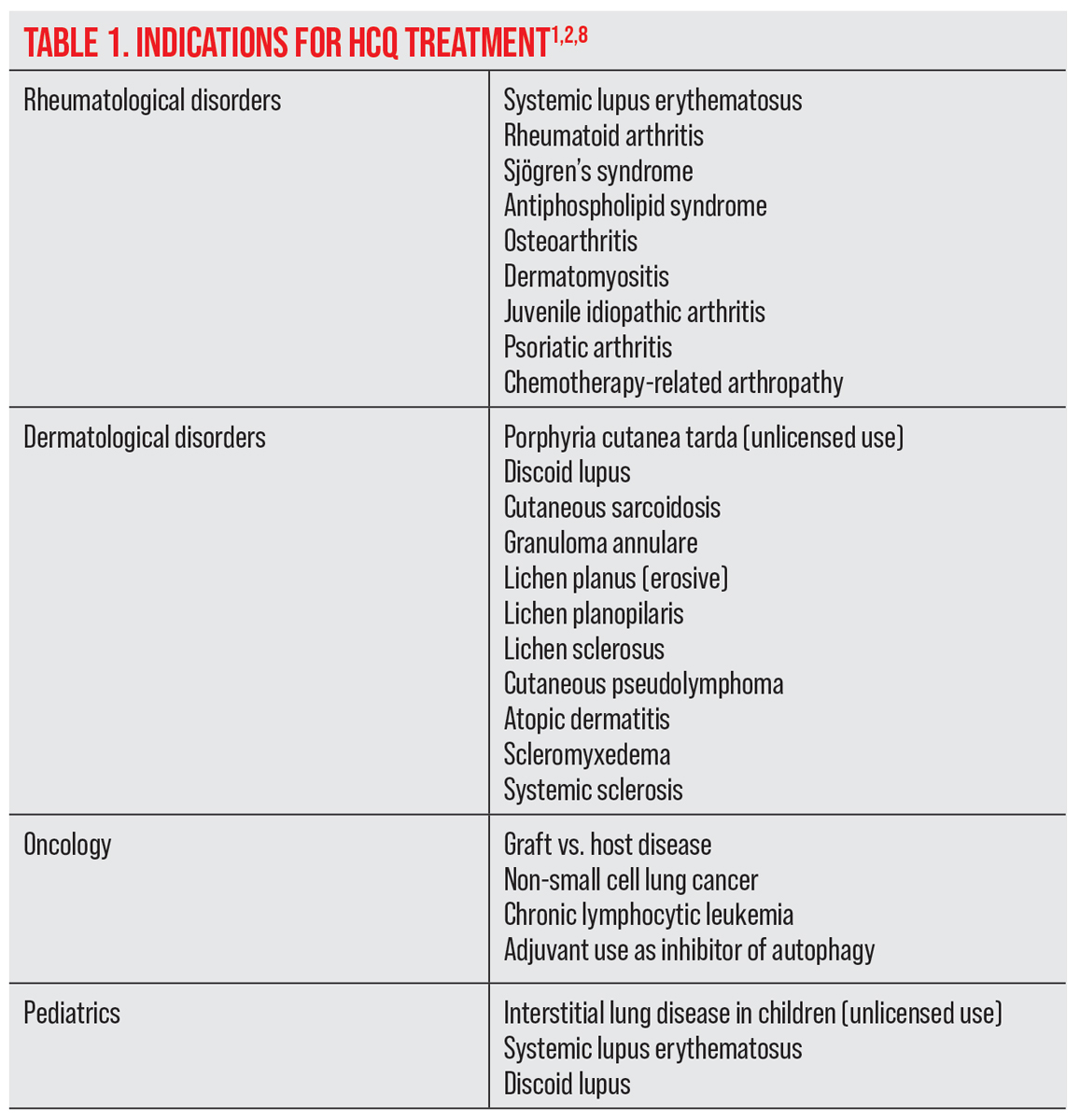
Plaquenil (hydroxychloroquine) is a disease-modifying anti-rheumatic drug used to treat patients with various inflammatory conditions through modulation of the immune system. Despite its reputable safety profile, you could still end up with the occasional patient in your chair who develops Plaquenil-induced toxicity, an untreatable condition that poses a serious threat to vision.
 |
|
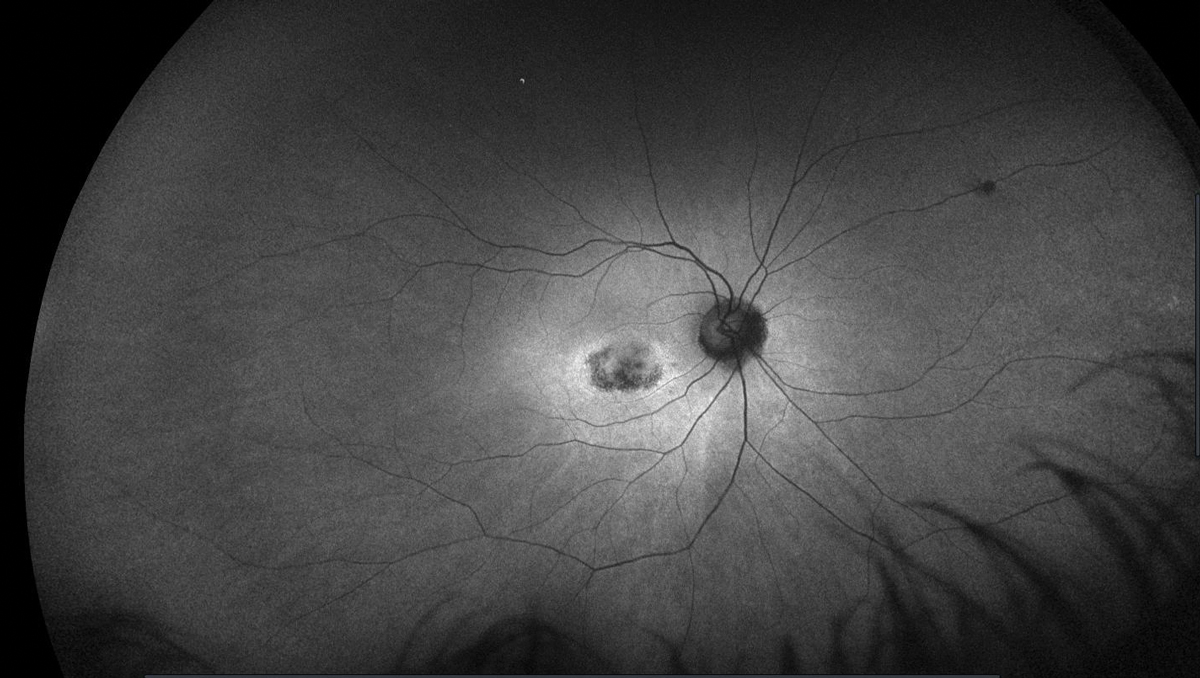
This image, taken five years after discontinuation of HCQ, shows abnormal FAF in the right eye of a patient who had been on the drug for over a decade. Click image to enlarge. |
Hydroxychloroquine (HCQ) was developed from its predecessor chloroquine in 1946 to be a more effective and less toxic alternative for the treatment of malaria.1-3 Its anti-inflammatory properties were recognized when improvement was noted in soldiers with autoimmune skin diseases and rheumatoid arthritis (RA).2,3 The agent is now commonly used in rheumatology for non-organ-specific autoimmune diseases, RA, mixed connective tissue disorders and related dermatological conditions in both adults and children.1,4,5 HCQ may also be beneficial for use in oncology, endocrinology (for diabetes) and cardiology.1,4,5 It may be used as an adjunct medication with biologic therapies, reducing the doses required of more toxic biologic drugs.1,6
 |
|
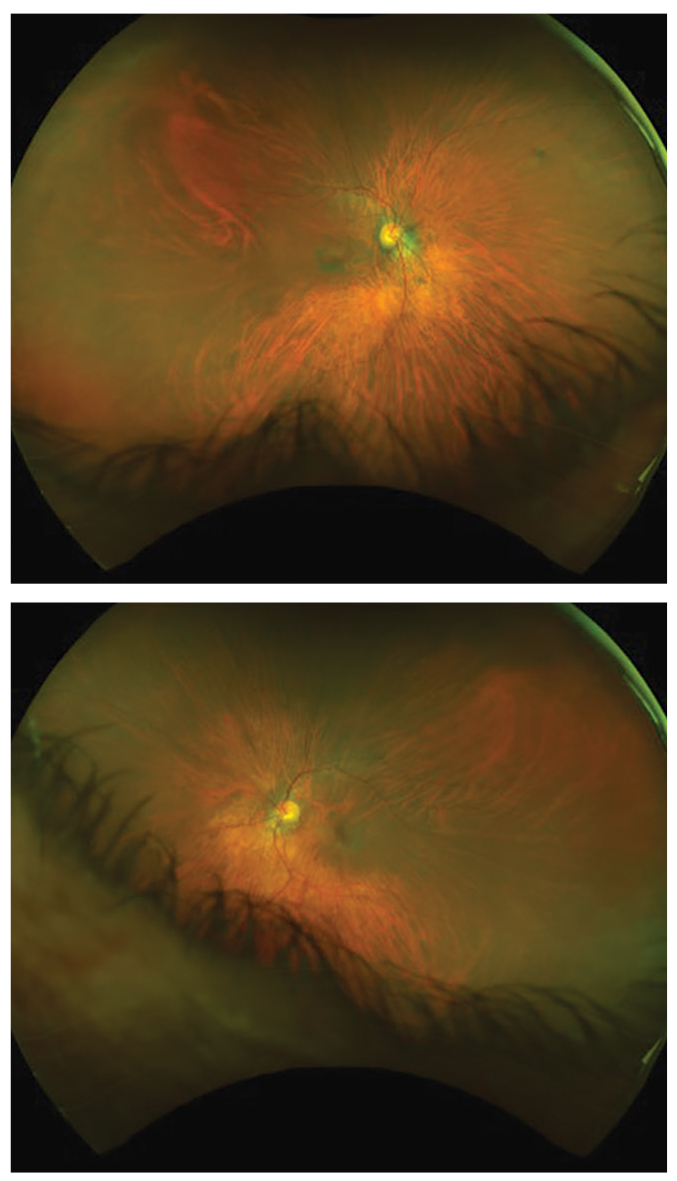
Five years after this patient stopped taking Plaquenil, the fundus images highlight some of the damage it left behind (OD, top; OS, bottom). Note the incomplete bullseye maculopathy OD. Click image to enlarge. |
HCQ has a lipophilic base, allowing it to cross cell membranes easily. This quality enables the suppression of antigen presentations, along with the production of prostaglandin and cytokine.7 The drug’s beneficial properties include antithrombotic, antifibrotic, antihyperglycemic, lipid reduction and immune suppressive properties.6,7 It lowers the risk of pregnancy complications in females who might require its use with systemic lupus erythematosus (SLE).6,8-10 Although the therapeutic is generally well tolerated, the adverse effects of HCQ include neuro-myotoxicity and cardiotoxicity in addition to its well-known risk for retinal toxicity.6,10
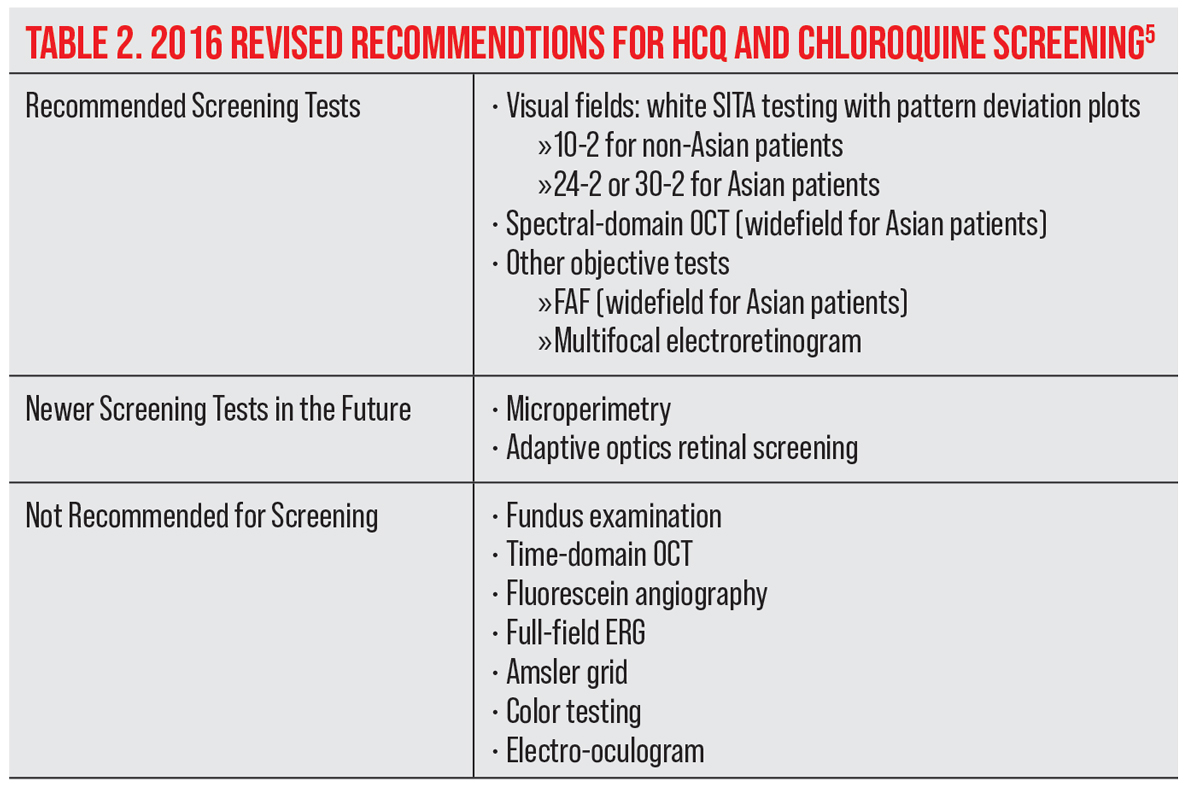
Screening guidelines for HCQ monitoring and testing strategies have changed over the past 20 years, helping us to better detect early retina changes before they are visible in the fundus. Visual field testing and spectral-domain optical coherence tomography (SD-OCT) are now the primary screening tests. Widefield testing is indicated for patients of Asian descent to detect the changes that develop more commonly beyond the central retina. New objective tests include multifocal electroretinogram (mfERG) to confirm visual field findings and fundus autofluorescence (FAF) to show fundus damage. Understanding the most recent guidelines allows us to identify those patients at high risk of toxicity, monitor for early signs of toxicity and discontinue medication before vision is affected.
A Toxic Case
For this 55-year-old, a negative outcome was avoided through proper and timely screening and intervention. The African-American female had presented to the eye clinic for a comprehensive examination and visual field testing. Her medical history was remarkable for episodic use of HCQ 400mg/daily from 1998 to 2012 and 200mg/day from 2014 to 2015, the second period of which was during the time of her visit.
The patient’s best-corrected visual acuities were 20/20 OD and 20/25 OS. Her external examination was normal, her confrontational visual fields were full and there was no afferent defect. Biomicroscopy uncovered normal anterior segment structures with Goldmann applanation pressures of 16mm Hg OU. A dilated fundus examination was remarkable for macular pigment mottling OU. SD-OCT showed loss of the IS/OS line and localized thinning of the outer nuclear layer, and FAF testing showed an area of hypo-fluorescence greater in the right eye than the left. Humphrey visual field (HVF) 10-2 showed defects in both eyes greater in the right than the left. These structural and functional changes were consistent with HCQ macular toxicity, and her rheumatologist was promptly notified that day with the recommendation to discontinue HCQ use. Subsequent yearly SD-OCT testing showed continued mild thinning of the outer nuclear layer and loss of the IS/OS line and increased hypofluorescence on FAF testing, demonstrating this drug’s ability to have lingering ocular effects even in less severe cases of toxicity.
 |
| Click table to enlarge. |
HCQ in Action: Mechanisms and Clinical Effects
HCQ is the mainstay of treatment in SLE, RA and a number of other related inflammatory rheumatic and dermatological conditions.2.4,7,10 It reduces the severity of SLE and its acute inflammatory events by 50%, delays the onset of SLE in patients with undifferentiated connective tissue disease and improves the overall survival rate of people diagnosed with the disease.2,6-10 It reduces the risk of congenital heart block in females with positive anti-Ro/SSA antibody and reduces the risk of seizures and thromboses.7,9 It is not recommended by the manufacturer for use during pregnancy and is listed as a schedule C pregnancy category medication.6,8 However, its use during pregnancy has been widely studied and has been shown to improve pregnancy outcomes in patients with SLE with no evidence of fetal harm.6,8,9
HCQ has a 70% to 80% bioavailability after oral administration with a half-life of 50 days, one characteristic that gives it the potential to cause lasting damage for up to several years even after drug discontinuation.2,6 Stable blood levels are reached within three to six months, and deposition in tissue may linger for as long as five years.2,6
 |
|
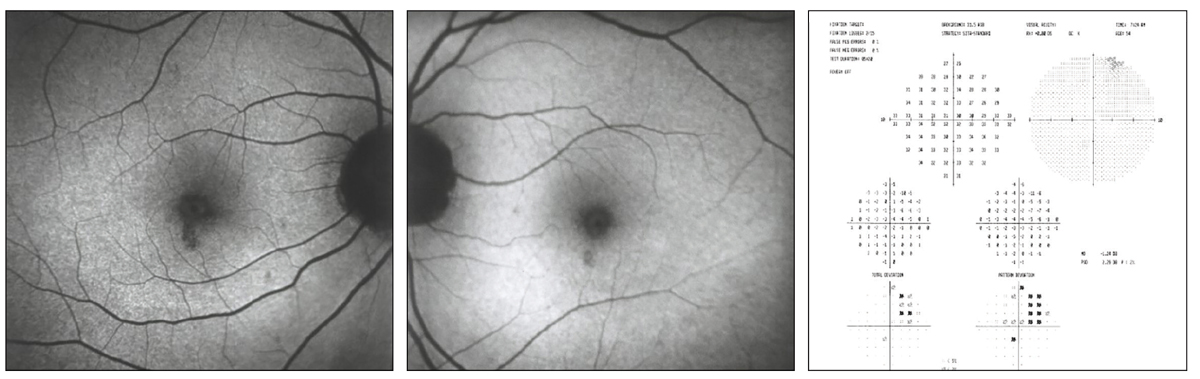
Initial visit FAF area of hypofluorescence (OD, left; OS, middle) indicating loss of RPE and corresponding to paracentral visual field defects 2’ HCQ toxicity (right). Click image to enlarge. |
The amount of drug measured in whole blood is almost five times higher than in plasma, making the assessment of therapeutic and toxic levels difficult.2,6 It is not retained in fatty tissue and is cleared predominantly by the kidneys (and to a lesser degree by the liver).1,5.11,12 The drug’s sneaky ability to remain in the body for an extended period of time is a key reason why early screening and monitoring these patients regularly to watch for signs of macular toxicity are so critical.
The common side effects of HCQ include gastrointestinal symptoms, nausea, diarrhea and vomiting that may resolve with continued use or require dose reduction.2,8,11,12 Cutaneous blue-gray pigmentations on the body are common, occurring in up to 25% of patients. Although pigmentation may persist, it is not associated with other adverse outcomes.6,8,11,12
Hemolytic anemia with glucose-6-phosphate dehydrogenase (G6PD) is a rare adverse effect that is only found in patients taking an amount that exceeds recommended doses; therefore, routine G6PD-deficiency screening is not indicated.6,8
Myopathy affecting the skeletal and heart tissue may occur but is rare.6,11,12 Myopathy will improve after HCQ is discontinued but may continue for weeks due to the long half-life of the drug.6,8 Cardiac involvement is extremely rare and presents as restrictive myopathy or conduction abnormalities.6,8,11,12 Discontinuation of HCQ may result in recovery, but death has also been reported.6,8 Annual electrocardiograms have been suggested to monitor potential adverse cardiac events, but there are no current guidelines for cardiotoxicity screening.8
Although safer than chloroquine, HCQ possesses risk for toxic retinopathy and irreversible vision loss.2,3,6,13 Vortex keratopathy associated with drug deposition into the cornea occurs in less than 5% of individuals taking 400mg/day.3,7 Corneal deposits may appear within two to three weeks of starting treatment and usually resolve when the drug is discontinued. Drug deposition into the cornea is not an indicator for those at risk for retinal damage and is not associated with vision loss.3,5
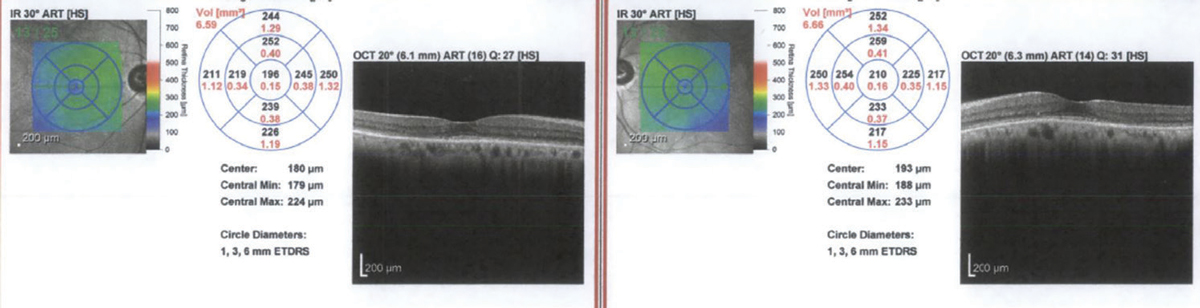 |
|
SD-OCT of the patient’s initial visit showing loss of the IS-OS junction and outer nuclear layer. Click image to enlarge. |
Retinal Toxicity
It is not clear why the photoreceptors in the parafoveal/perifoveal are susceptible to damage, and the mechanism of retinal toxicity is not well understood.1,3,4,17,18 HCQ strongly binds to tissues containing melanin, especially retinal pigment epithelial (RPE) cells.1,4,17,18 Some studies suggest that HCQ prevents lysosomal degradation and endocytosis of the RPE, which in turn stops the degradation of the old outer segments of photoreceptors. Additionally, HCQ’s binding to melanin may lead to delayed chronic toxicity, resulting in lipofuscin accumulation and impaired photoreceptor function.3,4,12,17,19-22
The outer segment of the photoreceptors is initially affected, followed by secondary degeneration of the RPE.1,4,12,17,19,20 Identifying early retinopathy before RPE damage occurs and discontinuing HCQ will limit progression and risk of vision loss. If early toxicity is not recognized or bullseye maculopathy is present at the time of exam, progression may continue for years with subsequent loss of vision despite having discontinued the drug.5,17
HCQ retinopathy occurs predominantly in the parafoveal area in Caucasians, Hispanics and African-Americans.5,23,24 A pericentral area or mixed pattern may rarely occur in Hispanics and African-Americans.5,23,24 Patients with retinopathy may initially be asymptomatic.2,16 The fundus appearance can look normal despite the presence of a scotoma.16 Initial complaints occur as a result of central or paracentral scotomas affecting night vision, reading and other fine visual functions, metamorphopsia and glare.3,16 Early scotomas are more common superiorly within 10° of fixation and may enlarge and multiply. Reduced visual acuity occurs if fixation becomes involved.16
Irregularity of macular pigmentation and decreased foveal reflexes are the earliest retinal findings.16 A concentric, horizontally oval area of hypopigmentation develops greater inferiorly and surrounds the macular irregularity.16 The paracentral depigmentation develops into the characteristic bullseye maculopathy pattern which signifies advanced severe damage.16 Generalized pigmentary changes may progress for a minimum of three years with continued thinning of the fovea and loss of EZ length and architecture despite cessation of the drug making it difficult to distinguish from cone-rod dystrophy.14,16-18
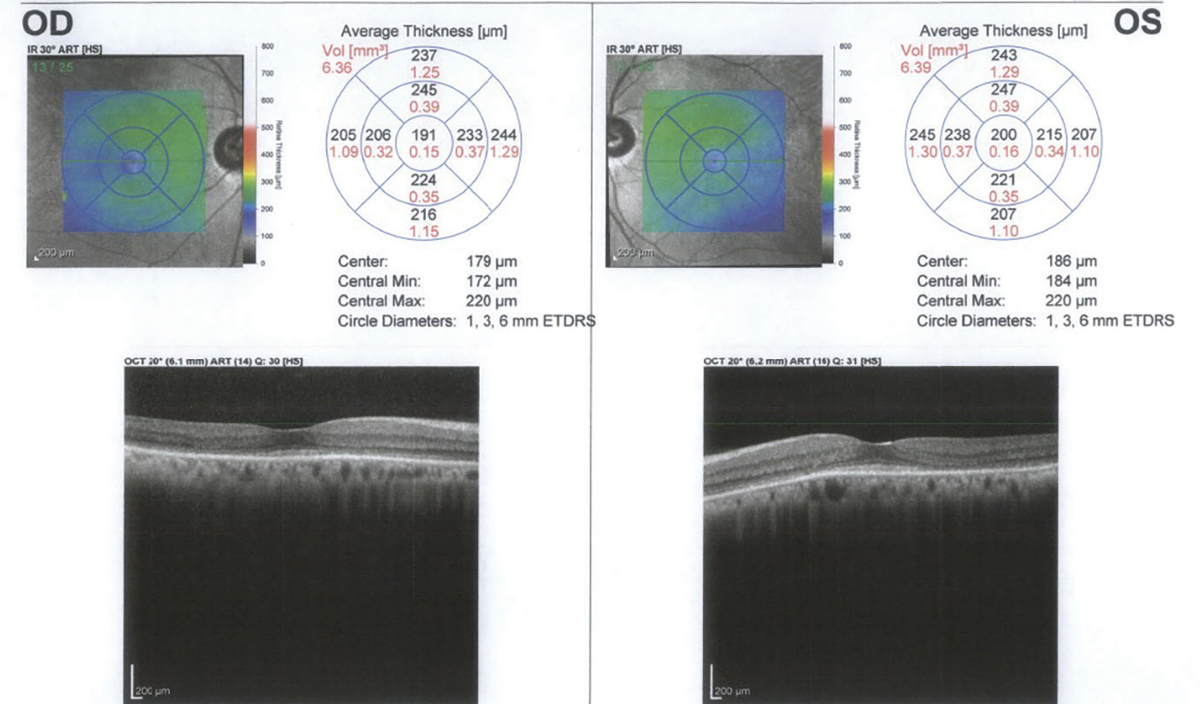 |
|
SD-OCT one year after discontinuation of HCQ showing continued loss of the IS-OS junction and outer nuclear layer. Click image to enlarge. |
Improper Dosing and Other Risk Factors
The 2011 American Academy of Ophthalmology (AAO) guidelines identified a cumulative dose of >1000g for increased risk of retinopathy.1,15 The 2011 guideline recommended that the daily dose of HCQ be no more than 400mg and that lower doses be calculated based on ideal body weight in the range of 6.5mg/kg for thin patients and those of short stature.14,15 It was also noted that obese patients can be dosed based on height if weight is not known.15
In 2016, the AAO revised the 2011 HCQ/chloroquine screening standards in response to new scientific data that identified specific risk factors for toxicity.5 A retrospective case-controlled study by Melles and Marmour that included 2,361 patients who used HCQ for at least five continuous years demonstrated a higher risk of retinal toxicity based on daily dose, duration of use and kidney disease.5,6,13 Risk factors identified included HCQ daily dose >5mg/kg actual body weight, use for greater than five years, reduced glomerular filtration rate, concurrent use of tamoxifen and pre-existing maculopathy.1,5
Although patients older than 60 years were previously thought to be at a higher risk of retinal toxicity, studies suggested that age alone was not a risk factor.5,13 However, since elderly patients are more likely to have decreased renal function or macular disease, their risk of toxicity is increased.1,5,13 Additionally, very thin patients were identified as having increased risk when dosing was based on ideal body weight.5,13
By identifying the retinal changes that occur earlier than the classic bullseye maculopathy pattern, researchers found a 7.5% risk for patients using long-term HCQ therapy, which is three times higher than the risk previously reported.10,13 The authors suggested a revised dose calculation to ≤5mg/kg/day using actual body weight, but no more than 400mg total daily.5,6,13 The subsequent risk of toxicity at this recommended dose is <1% up to five years, ≤2% at 10 years but then increases to almost 20% after 20 years.5 A patient who has no toxicity after 20 years has approximately a 4% risk of converting in the subsequent year.5,13
In addition to dosage, other factors increase the risk of toxicity. Kidney disease markedly increases the risk of retinopathy.1,2,5,13 A 50% decrease in kidney glomerular filtration rate will double the risk of ocular toxicity; the prevalence increases to greater than 50% in doses higher than 5mg/kg when used longer than 20 years.1,2,5,13 While HCQ is partially cleared by the liver, organ disease has not been reported to increase the risk of retinal toxicity.1,2,5 Tamoxifen, a non-steroidal estrogen antagonist used in the treatment of breast cancer, has its own risk of toxic retinopathy.11,13,15,16 The use of both HCQ and tamoxifen has an adverse synergy which increases the risk of retinopathy.13 Patients who are on both medications are at significantly higher risk of developing toxic retinopathy.1,2,11,13,16
A comprehensive medical history to review for the above risk and calculating the HCQ mg/kg actual (or real) body weight at each eye examination will provide the information needed to decide how often your patient should return for testing.
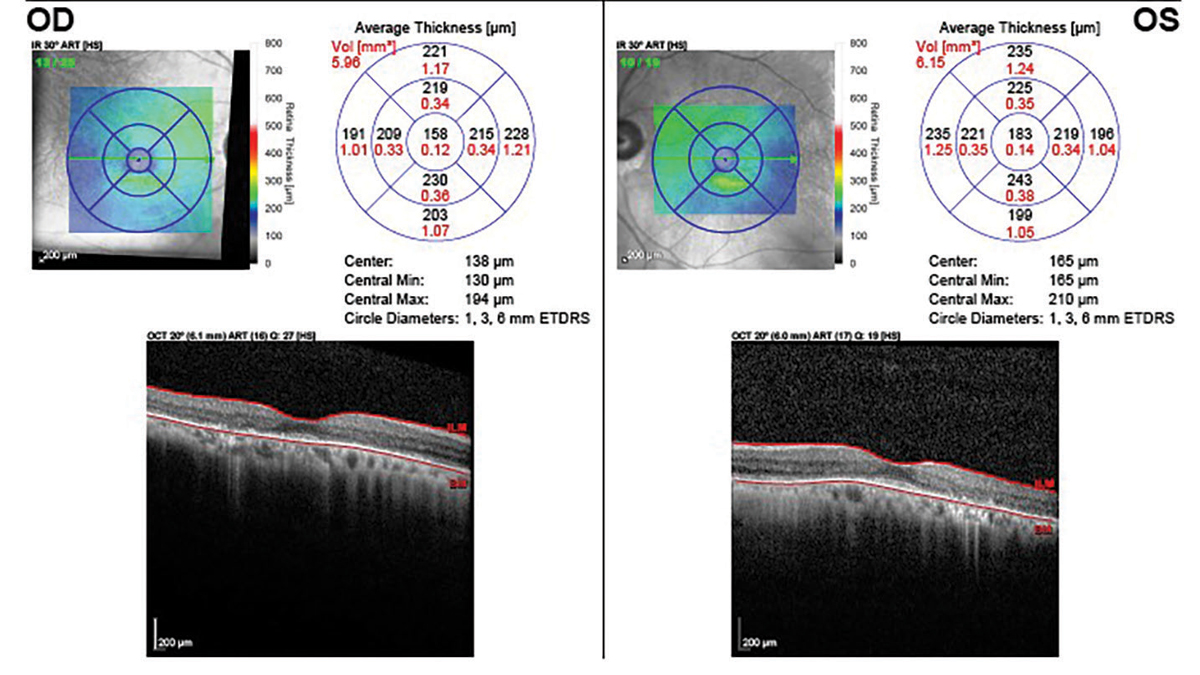 |
|
Five years after discontinuation showing loss of the IS-OS junction, outer nuclear layer and RPE. Click image to enlarge. |
Screening Guidelines
The AAO’s revised guideline for HCQ screening from 2016 included automated visual field testing, SD-OCT, FAF and mfERG.5 All patients should have a baseline retinal exam within the first year of administration to evaluate for other pre-existing ocular conditions or tissue damage that might interfere with the interpretation of screening tests.5 Baseline visual fields and SD-OCT are useful but not critical unless retinal abnormalities are present.5
Annual screening may be deferred until five years of drug use in those patients who have no major risk factors such as pre-existing ocular disease, maculopathy, concurrent tamoxifen use, renal disease or taking a dose >5mg/kg actual body weight.5 More frequent screening is recommended when major risk factors are present, but for every patient, it is important to check the dose relative to weight at every visit.
Routine screening recommendations include both objective (SD-OCT) and subjective (visual fields) testing. A white SITA 10-2 visual field with pattern deviation is recommended and preferred over the red isopters. Early damage occurs more frequently infratemporal with a corresponding superonasal field defect. Questionable results on visual fields should be repeated for consistency of loss and ideally confirmed with objective testing (SD-OCT, FAF or mfERG) before suggesting that treatment be altered or discontinued.5 However, treatment may be discontinued if consistent visual field defects are attributed to HCQ use.
Retinal damage on the SD-OCT is seen as loss of the IS/OS junction line and parafoveal thinning of the outer nuclear layer.14 FAF can show increased autofluorescence in early parafoveal or extramacular photoreceptor damage before thinning can be observed on SD-OCT. Late RPE loss will be dark in appearance, indicating reduced autofluorescence.5 The mfERG is the most sensitive objective test and may detect early signs of toxicity prior to visual or structural changes on OCT.2,5,26 However, its availability is often limited to specialized clinics.2,5
 |
| Click table to enlarge. |
A pericentral pattern involving the peripheral extramacular retina near the arcades is common in the Asian population.23,24,27 The mechanism for this finding is unclear, but it is more likely to be missed using only standard screening methods.6,13,23,27 Recommended adjustments for Asian patients include SD-OCT wide volume scans (12x9mm) or 30° scans for the purpose of being able to capture peripheral ellipsoid loss.4,23,27 An ultra-widefield FAF will capture 200° or 82% of the retina in contrast to the 30° to 50° evaluated on a conventional FAF.23,27 In addition to a 10-2 visual field, a 30-2 visual field would be appropriate for use on these patients to capture the more peripheral defects.23,24,27
Future screening tests may include OCT angiography, microperimetry and adaptive optics retinal imaging.21,25,28 Additionally, further development in genetic testing may help us eventually be able to identify those patients with a predisposition to a higher risk of retinal toxicity.5,9,29
These revised guidelines provide optometrists with the information needed to develop appropriate management plans and testing strategies for patients based on daily dose, cumulative dose, pre-existing ocular and systemic disease, concurrent medication risk and nationality.
Table 3. Dosing GuidelinesDose: maximum daily HCQ use of ≤5mg/kgactual (real) body weight, e.g.: 237 pounds = 107.5kg 400mg/107.5kg = 3.72mg/kg |
Clinical Takeaways
HCQ retinal toxicity is not treatable and is characterized by irreversible damage to the photoreceptor and/or RPE when the drug is taken for a long enough time that toxic levels of the compound build up in retinal tissues.3,13,23 Toxicity is not rare with patients on long-term therapy, and the risk is highly dependent on the daily dose, duration of dose as well as major systemic risk factors such as renal disease and concurrent use of tamoxifen. Pre-existing retinal and macular disease makes accurate interpretation of the screening tests more challenging to identify early toxicity suggesting that HCQ use be avoided in these patients. Lesser risk factors include age, liver disease and genetics, which all merit consideration though studies do not attribute a significant contribution to toxicity from these factors.5
Recommended dosing guidelines changed in 2016 and now state to use actual (real) body weight instead of ideal body weight to reduce risk of overdose, especially for thin patients.5,13 Lower risk occurs with doses ≤5mg/kg actual body weight. Patients of Asian descent may show early ellipsoid loss more peripherally and earlier in the arcade region rather than centrally.4 Studies show that 55% of Asian patients vs. 2% of Caucasian patients will develop a peripheral pericentral toxicity pattern instead of the well-known parafoveal pattern.4 For these patients, testing strategies must include the use of a widefield OCT, widefield FAF and enlarged visual field testing (24-2 or 30-2).23,24
With appropriate screening, central vision can be protected before changes occur in the RPE.5,13,18,24 Be sure to consistently question patients on their use of medication and know when screening is necessary to detect for disease before it’s too late.
Dr. Chubb is currently employed as a staff optometrist at the Michael J. Crescenz VA Medical Center in Philadelphia. She is also a fellow of the American Academy of Optometry. Dr. Chubb has no financial interests to disclose.
1. Yusuf IH, Sharma S, Luqmani R, Downes SM. Hydroxychloroquine retinopathy. Eye (Lond). 2017;31(6):828-45. 2. Abdulaziz N, Shah AR, McCune WJ. Hydroxychloroquine: balancing the need to maintain therapeutic levels with ocular safety: an update. Curr Opin Rheumatol. 2018;30(3):249-255. 3. Tehrani R, Ostrowski RA, Hariman R, Jay WM. Ocular toxicity of hydroxychloroquine. Sem Ophthalmol. 2008;23(3):201-9. 4. Corradetti G, Violanti S, Au A, Sarraf D. Wide-field retinal imaging and the detection of drug-associated retinal toxicity. Int J Retin Vitr. 2019;5(26). 5. Marmor MF, Kellner U, Lai TYY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmol. 2016;123(6):1386-94. 6. Shippey EA, Wagler VD, Collamer AN. Hydroxychloroquine: an old drug with new relevance. Cleve Clin J Med. 2018;85(6):459-67. 7. Ding HJ, Denniston AK, Rao VK, Gordon C. Hydroxychloroquine-related retinal toxicity. Rheum. 2016;55(6):957-67. 8. Fernandez AP. Updated recommendations on the use of hydroxychloroquine in dermatologic practice. J Am Acad Dermatol. 2017;76(6):1176-82. 9. Petri M, Elkhalifa M, Li J, et al. Hydroxychloroquine blood levels predict hydroxychloroquine retinopathy. Arthritis Rheumatol. 2020;72(3):448-53. 10. Jorge AM, Melles RB, Zhang Y, et al. Hydroxychloroquine prescription trends and predictors for excess dosing per recent ophthalmology guidelines. Arthritis Research and Therapy. 2018(20):133. 11. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Rheum. 2020;16(3):155-66. 12. Nirk EL, Reggiori F, Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med. 2020;12(8):e12476. 13. Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132(12):1453-60. 14. Mititelu M, Wong BH, Brenner M, et al. Progression of hydroxychloroquine toxic effects after drug therapy cessation: new evidence from multimodal imaging. JAMA Ophthalmol. 2013;131(9):1187-97. 15. Marmor MF, Kellner U, Lai TYY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmol. 2011;118(2):415-22. 16. Ringeisen AL, Mititelu M. Retinal toxicity of systemically administered drugs. In: Opthalmology. 5th ed. Elsevier. 2019. 17. deSisternes L, Hu J, Rubin DL, Marmor MF. Localization of damage in progressive hydroxychloroquine retinopathy on and off the drug: inner versus outer retina, parafovea versus peripheral fovea. IOVS. 2015;56(5):3415-26. 18. Marmor MF, Hu J. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 2014;132(9):1105-12. 19. Cakir A, Ozturan SG, Yildiz D, et al. Evaluation of photoreceptor outer segment length in hydroxychloroquine users. Eye (Lond). 2019;33(8):1321-6. 20. Bulut M, Kazim M, Toslak D, et al. A new objective parameter in hydroxychloroquine-induced retinal toxicity screening test: macular retinal ganglion cell-inner plexiform layer thickness. Arch Rheumatol. 2017;33(1):52-8. 21. Bulut M, Akidan M, Gözkaya O, et al. Optical coherence tomography angiography for screening of hydroxychloroquine-induced retinal alterations. Graefes Arch Clin Exp Ophthalmol. 2018;256(11):2075-81. 22. Lee MG, Kim SJ, Ham D, et al. Macular retinal ganglion cell-inner plexiform layer thickness in patients on hydroxychloroquine therapy. IOVS. 2015;56(1):396-402. 23. Ahn SJ, Joung J, Lim HW, Lee BR. Optical coherence tomography protocols for screening of hydroxychloroquine retinopathy in Asian patients. Am J Ophthalmol. 2017;184:11-18. 24. Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmol. 2015;122(1):110-6. 25. Dahrouj M, Young L. Hydroxychloroquine retinopathy in the era of advanced imaging modalities. Int Ophthalmol Clin. 2020;60(1):73-83. 26. Chang WH, Katz BJ, Warner JEA, et al. A novel method for screening the multifocal electroretinogram in patients using hydroxychloroquine. J Retina Vit Dis. 2008;28(10):1478-86. 27. Ahn, SJ, Joung J, Lee BR. Evaluation of hydroxychloroquine retinopathy using ultra-widefield fundus autofluorescence: peripheral findings in the retinopathy. Am J Ophthalmol. 2020;209:35-44. 28. Goker YS, Ucgul AC, Tekin K, et al. The validity of optical coherence tomography angiography as a screening test for the early detection of retinal changes in patients with hydroxychloroquine therapy. Curr Eye Res. 2019;44(3):311-15. 29. Wang G, Zhuo N, Liao Z, Qi W. Retinal toxicity caused by hydroxychloroquine in patients with systemic lupus erythematosus: a case report. Med (Baltimore). 2021;100(22):e25688. |

