A 56-year-old white male with a longstanding history of glaucoma presented for a consultation. He had not used any therapy for the past several years. He became frightened when the last doctor he saw recommended surgery. Since then, he had abandoned all medical treatment for his glaucoma and was in a state of denial.

His best-corrected visual acuity was 20/40 O.D. and 20/20 O.S. He had a relative afferent pupillary defect and constricted confrontation fields in his right eye. His intraocular pressure (IOP) was 48mm Hg O.D. and 28mm Hg O.S. There were no biomicroscopic abnormalities in either eye.
On gonioscopy, his anterior chamber angles showed complete appositional closure in each eye. An undilated fundus evaluation revealed advanced glaucomatous disc damage in his right eye and moderate damage in his left eye. He was diagnosed with chronic angle closure O.U. and was prescribed a prostaglandin analog (PGA) to be used in each eye at bedtime.
When he returned for follow-up six days later, he reported medical compliance and tolerance of the medication without adverse effects. At this visit, his IOP measured 21mm Hg O.D. and 14mm Hg, which was deemed an outstanding medical response. Currently, he is awaiting insurance clearance for a laser iridotomy.
Is There a Role for PGAs in Angle-Closure Glaucoma?
Prostaglandin analogs work by increasing the flow of aqueous out of the eye via the uveoscleral meshwork. Most clinicians consider these drugs to be a first-line therapy for patients with primary open-angle glaucoma (POAG). However, there is much confusion as to what effect, if any, PGAs have on other forms of glaucoma.
To be sure, prostaglandins typically are avoided in patients with concurrent primary inflammation due to the potential to increase inflammation. But, what about patients with angle closure?
• Acute angle closure. Medical management of acute angle-closure glaucoma is well known and consists of aqueous suppressants, miotics and, ultimately, laser peripheral iridotomy (LPI) to break the pupil block and open the anterior chamber angle.
However, little is known about the role of PGAs in the management of acute angle-closure glaucoma. The general clinical consensus seems to be that PGAs are not indicated in the management of an acute angle-closure glaucoma attack. While there is no evidence that PGAs are detrimental in these cases, it is believed that the onset of pressure reduction action is generally unacceptably slow to be of any value in an acute situation.1
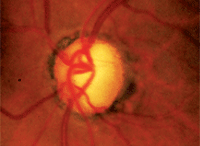
Advanced glaucomatous disc damage, as often seen in patients who present with angle-closure glaucoma. Could prostaglandin analog
therapy help to reduce further damage?
• Chronic angle closure. In contrast to acute angle closure, much is known about the effects and role of PGAs in chronic angle-closure glaucoma (CACG). In primary CACG there are anatomical features acting in concert that cause shallowing of the anterior chamber. As a patient ages, thickening of the crystalline lens leads to a relative pupil block that exacerbates the condition. This all acts to put the iris into apposition with the trabecular meshwork. Because the closure is slow, there is an absence of symptoms that would typify an acute angle closure.
Chronic appositional closure often will lead to peripheral anterior synechiae (PAS) if untreated. While there is asymmetric closure involving first the superior angle in most cases, there also can be a steady circumferential closure that slowly progresses symmetrically. This has been termed “creeping angle closure,” and appears as an angle that becomes progressively more shallow over time.2,3
Management Strategies
Management of chronic angle closure virtually always entails LPI. However, due to trabecular damage from the appositional chronic angle closure or the persistence of PAS, IOP is often elevated even after successful LPI, necessitating subsequent chronic medical therapy.
Jin-Wei Cheng, M.D., and associates conducted a meta-analysis of patients with CACG who were treated with PGAs and found that travoprost, latanoprost and bimatoprost significantly lowered IOP in eyes with CACG; all were at least as effective as timolol.4 Another study showed bimatoprost and latanoprost to be similar in IOP reducing efficacy in eyes with CACG.5
Rajesh S. Kumar, M.D., and colleagues noted that latanoprost significantly lowered IOP in eyes with CACG, and that the IOP-lowering effect was not influenced by the degree of PAS present.6
Additionally, one other study noted successful IOP reduction with both latanoprost and bimatoprost in eyes with CACG that were unsuccessfully managed by LPI alone.7
There have also been numerous reports indicating the success of PGAs in eyes with CACG.8-10 In many of these studies, the pupil block component had been removed with LPI and––in many cases––the angles were effectively opened.11
More fascinating is the effect of PGAs in eyes with actual closure and PAS. Tin Aung, M.D., and members of the EXACT Study group saw that the excellent IOP lowering effect of latanoprost was not, in fact, impacted by the degree of angle closure or the extent of synechial closure.12
Another study of eyes with CACG and 360° of ultrasound biomicroscopically- and gonioscopically-proven PAS remarkably found that treatment with latanoprost once-daily resulted in a significant reduction in IOP.13 While PGAs are thought to lower IOP by increasing matrix metalloproteinase activity and reducing the amount of extracellular matrix material surrounding the ciliary muscle fiber bundles, the mechanism of PGA action in eyes with no visible uveoscleral meshwork or ciliary face is not well understood. It was theorized in this report that latanoprost possibly gained access to the ciliary body or other uveoscleral structures through a yet unidentified pathway that possibly involves the iris, and that outflow of aqueous may involve something other than uveoscleral meshwork in these eyes with complete PAS angle closure.13
Although PGAs are commonly used in POAG due to their excellent effect, it should be understood that this class of medication seems to work exceptionally well in eyes with CACG.
PGAs provide excellent IOP reduction in eyes that require medical therapy after LPI and are also very effective in eyes with near complete chronic angle closure––even in angles completely closed with PAS. Always remember the excellent effects of PGAs when encountering this particularly malignant form of glaucoma.
1. Hoh ST, Aung T, Chew PT. Medical management of angle closure glaucoma. Semin Ophthalmol. 2002 Jun;17(2):79-83.
2. Mok KH, Lee VW. Synechial angle closure pattern in Chinese chronic primary angle-closure glaucoma patients. J Glaucoma. 2001 Oct;10(5):427-8.
3. Lowe RF. Primary creeping angle closure glaucoma. Br J Ophthalmol. 1964 Oct;48:544-50.
4. Cheng JW, Cai JP, Li Y, Wei RL. A meta-analysis of topical prostaglandin analogs in the treatment of chronic angle-closure glaucoma. J Glaucoma. 2009 Dec;18(9):652-7.
5. How AC, Kumar RS, Chen YM, et al. A randomised crossover study comparing bimatoprost and latanoprost in subjects with primary angle closure glaucoma. Br J Ophthalmol. 2009 Jun;93(6):782-6.
6. Kumar S, Malik A, Singh M, Sood S. Efficacy of latanoprost in management of chronic angle closure glaucoma. Nepal J Ophthalmol. 2009 Jan-Jun;1(1):32-6
7. Chen MJ, Chen YC, Chou CK, Hsu WM. Comparison of the effects of latanoprost and bimatoprost on intraocular pressure in chronic angle-closure glaucoma. J Ocul Pharmacol Ther. 2007 Dec;23(6):559-66.
8. Gupta V, Srinivasan G, Sharma A, et al. Comparative evaluation of bimatoprost monotherapy in primary chronic angle closure and primary open angle glaucoma eyes: a three-year study. J Ocul Pharmacol Ther. 2007 Aug;23(4):351-8.
9. Chen MJ, Chen YC, Chou CK, Hsu WM. Comparison of the effects of latanoprost and travoprost on intraocular pressure in chronic angle-closure glaucoma. J Ocul Pharmacol Ther. 2006 Dec;22(6):449-54.
10. Sakai H, Shinjyo S, Nakamura Y, et al. Comparison of latanoprost monotherapy and combined therapy of 0.5% timolol and 1% dorzolamide in chronic primary angle-closure glaucoma (CACG) in Japanese patients. J Ocul Pharmacol Ther. 2005 Dec;21(6):483-9.
11. Wang N, Fan Z, Wu H, et al. Drug therapy for residual angle-closure glaucoma after laser iridectomy. Zhonghua Yan Ke Za Zhi. 2002 Dec;38(12):712-6.
12. Aung T, Chan YH, Chew PT; The EXACT Study Group. Degree of angle closure and the intraocular pressure-lowering effect of latanoprost in subjects with chronic angle-closure glaucoma. Ophthalmology. 2005 Feb;112(2):267-71.
13. Kook MS, Cho HS, Yang SJ, et al. Efficacy of latanoprost in patients with chronic angle-closure glaucoma and no visible ciliary-body face: a preliminary study. J Ocul Pharmacol Ther. 2005 Feb;21(1):75-84.

