 |
Diabetic retinopathy (DR) is the leading cause of vision loss globally in the working, middle-aged adult population.1 Nearly all patients with insulin-dependent diabetes develop vascular complications within 15 years of diagnosis, and approximately 34.6% of patients with diabetes exhibit some form of retinopathy.1,2 Though the precise pathophysiologic mechanism of DR is not completely understood, it has long been associated with damage to the retinal vasculature and loss of pericytes specifically.3,4
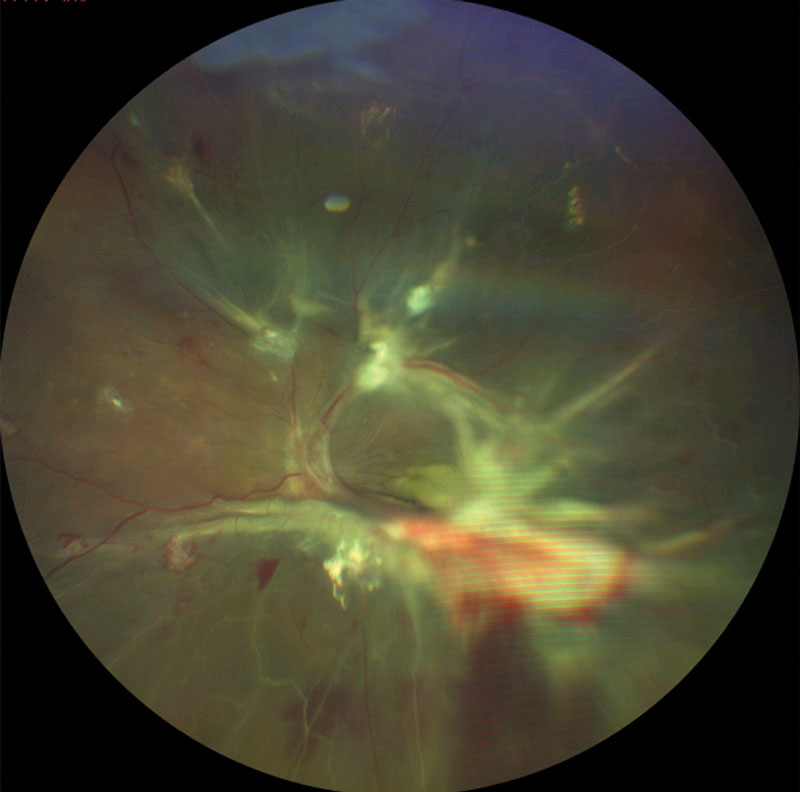 |
| This patient with proliferative DR shows signs of severe vascular dysregulation. |
High Demand
The oxygen requirement for the retina is the highest of all tissues in the human body, exceeding even the brain. To accommodate this demand, the retina requires an extensive—and intact—vascular network to obtain the oxygen and nutrients necessary for adequate function. As such, the retina is supplied by two vascular systems: the central retinal artery nourishes the inner retinal layers, while the choroidal capillaries nourish the outer retinal layers.3
Damage from DR, observed at the microvascular level, is split into two categories: non-proliferative and proliferative. Early, or non-proliferative, DR manifests clinically as retinal microaneurysms or microhemorrhages. In later stages, proliferative DR appears as neovascular growth from existing retinal vessels.3 One of the earliest abnormalities in the diabetic retina, leading to these microvascular aneurysms and hemorrhages, is pericyte dropout.4
The Pericyte-Glaucoma ConnectionThe correlation between pericyte loss and the manifestation of DR is well documented; in fact, DR is perhaps the most studied pathological condition resulting from pericyte loss. However, the impact of pericyte loss has also been implicated as a possible pathological mechanism in glaucoma development. Similar to the mechanisms involved in DR, vascular dysregulation and increased blood-retina barrier breakdown at the level of the optic nerve head can contribute to glaucomatous disease and progression. Further studies are warranted to determine the extent of pericyte involvement in glaucomatous optic neuropathy.5 |
Surface Support
Pericytes were first described in the 1870s by Eberth and Rouget and were initially named Rouget cells. They were later renamed “pericytes” because of their perivascular location relative to the endothelial cells. Pericytes are located on the surface of capillaries and function primarily to stabilize and support the blood vessels. They arise from neuroectodermal neural crest cells and have the highest density in the central nervous system.5
Identifying pericytes is often difficult because their cytoplasm may span several endothelial cells and exhibit different morphologies. As such, investigators have studied histological methods, revealing pericyte-specific markers that can aid with recognition. These include platelet-derived growth factor receptor β, NG2, CD13, desmin and vimentin.4
Pericytes in the retina are not readily observed on routine testing; they are approximately 7µm and extend distal processes that are even smaller, limiting the use of conventional imaging techniques. Experimental methods have included in vitro and post-mortem histological examinations. More recently, a study using adaptive optics scanning laser ophthalmoscopy showed success in visualizing the retinal cellular structure in vivo using mouse models.6
Pericytes in the adult retina work as regulators of gene expression to protect the retinal vessels from stress and injury.4 In healthy eyes, the endothelial cell-to-pericyte ratio is 1:1. In diabetic retinas, this ratio drops to 4:1. Prolonged hyperglycemia and genetic factors in diabetics lead to DR damage through several mechanisms, such as activation of protein kinase C, activation of the polyol pathway, non-enzymatic glycation, inflammation and oxidative stress. These events trigger pericyte death through apoptosis and dropout, leading to retinal microaneurysms, vessel tortuosity, hyperpermeability and capillary non-perfusion.2,3
Studies investigating pericytes in mice found that those with reduced numbers of pericytes experienced severe hemorrhage. Furthermore, mice with less than 52% of normal pericyte density developed signs of proliferative DR.5 Microaneurysm formation in DR is partially due to the over-proliferation of pericyte-deficient endothelial cells in response to vascular endothelial growth factor from hypoxia.2
Research also suggests that pericyte loss from retinal capillary walls is responsible for the breakdown of the inner blood-retina barrier (BRB). While research shows that the tight junctions of the endothelial cells and the retinal pigmented epithelial cells make up the inner and outer BRB, many studies have concluded that reduced pericyte numbers also lead to increased permeability and BRB breakdown.5
Valuable and Vulnerable
Pericytes—small, specialized cells on the surface of capillaries—have a significant impact on vascular homeostasis. A better understanding of their prominent role in the pathogenesis of DR, and possibly other ocular diseases, can help spur the development of potential therapeutic targets to delay or treat this leading cause of vision loss.
| 1. Kusuhara S, Fukushima Y, Ogura S, et al. Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab J. 2018;42:364-76. 2. Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: breaking the barrier. Pathophysiology. 2017;24(4):229-41. 3. Arboleda-Velasquez JF, Valdez CN, Marko CK, D’Amore PA. From pathobiology to the targeting of pericytes for the treatment of diabetic retinopathy. Curr Diab Rep. 2015;15(2):1-19. 4. Santos GSP, Prazeres PHDM, Mintz A, Birbrair A. Role of pericytes in the retina. Eye. 2018;32:483-86. 5. Trost A, Lange S, Schroedl F, et al. Brain and retinal pericytes: origin, function and role. Frontiers in Cellular Neuroscience. 2016;10(20):1-13. 6. Shallek J, Geng Y, Nguyen H, Williams DR. Morphology and topography of retinal pericytes in the living mouse retina using in vivo adaptive optics imaging and ex vivo characterization. Invest Ophthalmol Vis Sci. 2013; 54(13):8237-50. |

