Back in the 1980s, a “glaucoma workup” typically consisted of perimetry testing, gonioscopy, detailed hand drawings of the optic nerve and multiple IOP measurements. Our understanding of glaucoma has evolved significantly over the intervening years and, thanks to advances in technology, we can now achieve a much finer-grained assessment of ocular structures. Currently, the standard of care for glaucoma evaluation includes pachymetry, nerve fiber layer and optic nerve analysis, as well as evaluation of blood pressure. That list may soon expand to include other assessments, including a nutritional analysis and possibly even genetic testing.
Here, we explore the relationship between nutrition and glaucoma and its possible impact on your patients and your practice.
Intraocular Pressure
Evidence that elevated IOP increases the chance of glaucomatous optic nerve damage is plentiful.1-4 For example, the Early Manifest Glaucoma Trial (EMGT) shows that the hazard ratio for progression increased by 11% for every 1mm Hg increase in IOP.5 The Barbados Eye Study found that among persons who developed glaucoma, nearly half (46%) had baseline IOPs greater than 22mm Hg. Similar to the trends found in the EMGT, the Barbados Eye Study also found that the risk of open angle glaucoma (OAG) increased by 12% with each 1mm Hg increase in IOP. For persons with baseline IOP under 17mm Hg, incidence of OAG was 1.8%, but for those with baseline IOP greater than 25mm Hg, the incidence was 22.3%.2 Research shows that for a person whose IOP is above 21mm Hg, the risk of developing glaucoma is 16 times higher than for a person with IOP lower than 16mm Hg.6 High IOP is still the biggest risk factor to consider, but it’s not the only one.
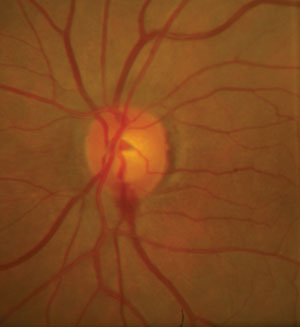 | |
| Nutritional substances may serve as adjuvant therapies for patients with glaucoma to help minimize hemorrhaging at the optic disc. Photo: Alan G. Kabat, OD. |
Other Factors
Despite the well-established link between increased IOP and glaucomatous nerve damage, it is certainly not an exclusive relationship. A significant proportion of patients with IOP greater than 21mm Hg do not ever develop glaucoma.6-10 Also, many patients with glaucoma don’t have an elevated IOP.6,10 In fact, up to one half of patients with glaucoma have IOP of 20mm Hg or lower at initial diagnosis.6,10 Close to 20% of patients in the Baltimore Eye Survey had an IOP of less than 21mm Hg on each of their first three glaucoma visits.11,12 Normal tension glaucoma (NTG) is even more common in Asian populations.13,14
Further, significant racial differences exist in the prevalence of glaucoma. The Baltimore Eye Survey reported that African Americans had a prevalence of primary open-angle glaucoma (POAG) that was 4.3 times higher than in Caucasians.11,15 Latinos older than age 60 have a risk of glaucoma development approximately the same as for African Americans.16
This leaves us wondering why some patients with IOP in the mid-20s suffer severe nerve damage, while others don’t. We had other questions too. Why do some patients with IOP in the high teens remain disease-free, while others with the same IOP suffer progressive field loss? Why do African Americans and Latinos suffer more severe nerve damage than Caucasians, even after correcting for central corneal thickness differences? Why do some patients have an aggressively progressive form, while others seem to have a more stable or even non-progressive profile?
Non-IOP Mechanisms
Clearly, the relationship between IOP and glaucomatous damage is not linear. Other mechanisms are indisputably involved in this complex disease. Vascular dysregulation and oxidative stress are two proposed mechanisms that may lead to glaucomatous ganglion cell apoptosis, or programmed cell death.17
Accumulation of advanced glycation endproducts (AGEs) within the retina and optic nerve head in glaucomatous eyes is another proposed mechanism in the pathogenesis of glaucoma. Increased rigidity of the lamina cribrosa is one possible direct consequence of AGE accumulation. The glaucomatous trabecular meshwork may also undergo earlier senescence under the influence of AGEs, compared with normal eyes.18
Alterations in glutamate and heat shock proteins are reported in glaucomatous eyes compared with normal eyes.19,20 Genetics almost certainly play a role in glaucoma development, and some evidence shows that nutritional deficiencies may also contribute.21,22 Decreased cerebrospinal fluid pressure is seen more commonly in patients with NTG, suggesting a possible connection. What is not entirely clear is whether each of these factors is individually to blame in different subsets of patients, or whether some—or all—of them combine and intertwine with each other to create a final common pathway culminating in glaucomatous visual field loss. Two of these proposed factors—vascular dysregulation and oxidative stress—may be modifiable with nutrition. Let’s look at these mechanisms in greater detail.
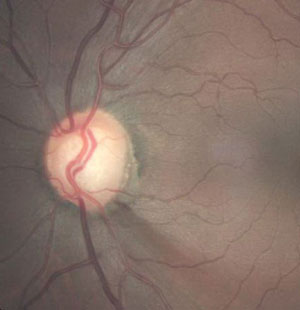 | |
| Glaucomatous optic disc hemorrhage and associated, adjacent RNFL defect in a glaucoma patient. Photo: Joseph W. Sowka, OD. |
Vascular Dysregulation
Vascular dysregulation, commonly defined as glaucoma with IOP consistently below 21mm Hg, is believed to be a significant contributor to glaucomatous progression in normal tension glaucoma.23-25 Although systemic hypotension is a recognized risk factor for NTG, investigators believe a more critical indicator is the diastolic perfusion pressure (DPP), which takes IOP into account. DPP is calculated as the difference between diastolic blood pressure and IOP. For example, a patient with systemic blood pressure of 110/65 and IOP of 15mm Hg would have a DPP value of 65-15=50. Values less than 55mm Hg have been associated with a two- to six-fold increase in glaucoma.26-27 The Barbados Eye Study found that lower systolic BP, and particularly lower ocular perfusion pressures, doubled the risk of glaucoma.28 A recent study compared the ocular blood flow of individuals of African descent with the ocular blood flow of individuals of European descent with open angle glaucoma, and found significantly lower blood flow values in all retrobulbar blood vessels in African descent patients.29
Regulation of blood flow in small vessels is accomplished in large part by two substances that are endogenously produced. Endothelin-1 (ET-1) is a potent vasoconstrictor, while nitric oxide (NO) is a vasodilator. Studies show both to be abnormal in glaucoma patients.30 One theory is that in susceptible patients, a delayed response to oxygen demand results in prolonged ischemia and an exaggerated reperfusion response. That reperfusion, rather than the initial hypoxia, is thought to be injurious to the optic nerve.31 Clinical “soft signs” which may represent visible manifestations of irregular ocular perfusion in these patients include beta zone peripapillary atrophy and optic disc hemorrhages, the latter of which herald a higher likelihood of visual field loss.32,33 Normalization of ET-1 and NO is an attractive, but elusive, target for pharmaceutical intervention.34
Oxidative Stress
Retinal ganglion cells require a great deal of energy compared with other tissues and cells. The mitochondria of these ganglion cells are responsible for their energy production, and consequently produce relatively larger amounts of reactive oxygen species (ROS), or free radicals—one of these being the superoxide radical. Endogenously produced superoxide dismutases (SOD-1, -2, and -3) are responsible for neutralizing superoxide. Zinc and copper are essential in the structure and function of SOD-1 and SOD-3, which function as an antioxidant within cell cytoplasm and in extracellular spaces, respectively. Manganese is required for SOD-2, which is thought to function exclusively in the mitochondrial space. A reduction in the quantity or quality of these enzymes has been linked to a variety of diseases, and mutations in genes coding for even a single amino acid related to SOD can have a devastating effect on health.35
Increased oxidative stress is implicated in open angle glaucoma.36-37 Assuming it does contribute significantly to the pathogenesis of the disease, it seems reasonable to assume that increasing total antioxidant capacity could provide protection in glaucoma patients. How to accomplish that is a therapeutic challenge.
Nutritional Strategies
Several nutritional substances have shown promise as adjuvant therapies in glaucoma by targeting the vascular dysregulation model, the oxidative stress model or both, of glaucoma pathogenesis.38-48
Ginkgo biloba
Ginkgo biloba is possibly the most-studied among nutritional therapies in glaucoma. In one study, ginkgo biloba, dosed at 40mg TID, was shown to improve blood flow to the peripapillary area.38 Ginkgo biloba likely has antioxidant properties as well.39 Importantly, ginkgo biloba—unlike currently available pharmacological agents— appears to function in both lipophilic and hydrophilic environments, allowing it to reach the inner mitochondrial membranes that are highly vulnerable to oxidative damage.40 Ginkgo biloba supplementation does carry a risk of adverse effects, the most serious of which is increased risk of bleeding. Other reported side effects include increased blood pressure when combined with thiazide diuretics and one case of coma in a patient taking the antidepressant trazodone.41
Black currant anthocyanins
Black currants contain a complex spectrum of anthocyanins and have been shown to normalize abnormal serum endothelin-1 levels in patients with glaucoma.42,43 Further, IOP-lowering effects may be achieved with oral administration of 50mg a day of black currant anthocyanins.44 A study also reported improvements in ocular blood flow and reduction in visual field progression compared to patients given placebo.45
Mirtogenol
This proprietary blend of standardized bilberry extract Mirtoselect and Pycnogenol, a French maritime pine bark extract, was shown to reduce IOP and increase systolic and diastolic ocular blood flow, thereby increasing the diastolic perfusion pressure.46 A study of 79 subjects in 2010 compared Mirtogenol alone with latanoprost alone, and a combination of both.47 Although both Mirtogenol and latanoprost alone were beneficial, a synergistic relationship was found in the combination group for intraocular pressure reduction as well as increases in systolic and diastolic blood flow, especially after several weeks of treatment.47 No side effects from Mirtogenol have been reported in the literature to date.
Manganese
A recent study found that blood manganese levels were negatively associated, while mercury levels were positively associated, with the odds of glaucoma diagnosis in a South Korean population.48 This is suggestive, but not conclusive, that low levels of manganese may contribute to the pathogenesis of glaucoma, possibly connected with reduced function of SOD-2 to neutralize superoxide at the level of the inner mitochondrial membrane.
Other nutrients
Several other substances have been studied less extensively, but may possibly show some promise in adjuvant therapy for glaucoma. These include resveratrol, magnesium, ubiquinone, melatonin and bilberry extract. Coffee is a well-known antioxidant and scavenger of the superoxide radical. Green, white and black teas all have antioxidant capacity and may potentially reach therapeutic levels at the retinal ganglion cells. Further study of these substances is warranted.49
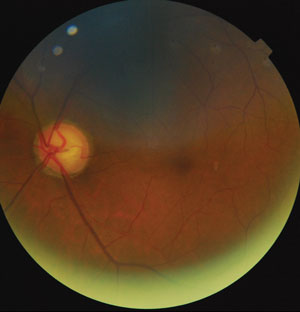 | |
| Optic nerve thinning due to glaucoma. Photo: Justin Cole, OD. |
Today’s Glaucoma Patient
Even without considering nutritional strategies, taking blood pressure on all patients with glaucoma is good clinical practice. Calculation of diastolic perfusion pressure may help to identify patients at risk for more rapid progression, prompting more aggressive treatment and follow up. In addition, it is especially important to consider these risks in hypertensive patients taking blood pressure-lowering medications, as exaggerated nocturnal hypotension must be avoided.50 On the other hand, patients who do not display a drop in blood pressure overnight, the so-called “non-dippers,” are significantly more at risk of death from a cardiovascular event than those who display a more typical diurnal curve. Modification of treatment regimens may need to be discussed with the patient’s other health care providers, with 24-hour blood pressure measurement, before medications are altered to avoid putting patients at systemic risk.
Ideally, patients with poor nutritional status could be readily identified and targeted for intervention. But how can you identify which patients need intervention? Should those patients, once identified, be advised to take a multivitamin/mineral supplement (MVM), or instead, take specific supplements targeted towards glaucoma?
Glaucoma Prevention in Practice
A good starting point is to do a baseline dietary analysis. This is sound clinical practice for all patients and can easily be done by a staff member prior to examination. A diet lower in fruits and vegetables, especially berries and dark leafy greens, is unquestionably associated with higher prevalence of a multitude of both systemic and ocular diseases. The recommendation to incorporate more of these whole foods into the diet, based on an informal food frequency survey, is theoretically ideal, but unfortunately not likely to be met with 100% compliance.
A slightly better approach is to capture indirect measures of nutrient levels, which can be done non-invasively in the office. Macular pigment optical density (MPOD) testing is a subjective test that is designed to measure xanthophyll (lutein and zeaxanthin) levels at the macula. Although xanthophylls have not, to date, been directly implicated in glaucoma in large clinical trials, this may serve as a proxy measurement of general nutrition intake, since foods containing lutein and zeaxanthin (spinach, kale, orange and yellow peppers, and pumpkin, to name just a few) also offer significant antioxidant properties and trace mineral levels. Transdermal nutrient testing gives an estimate of carotenoids in the skin. Both of these techniques can be easily performed by staff members, provide immediate results, establish a treatment baseline, and can increase compliance with your suggested supplementation regimen. Having a baseline and a target allows both practitioner and patient to monitor improvement and can be a strong motivator at follow-up visits. Bear in mind that these tests are estimating levels of only a portion of the substances that may ultimately improve a patient’s outcomes with respect to glaucoma, and do not offer a direct measure of nutrients more directly linked to glaucoma. Mineral levels—including manganese, magnesium and zinc—are not addressed.
Another method is to test the patient’s blood, serum or cellular membranes for specific nutrient profiles. Standard laboratory analysis is available for many individual nutrients, and several independent companies offer comprehensive nutritional testing. An advantage to the latter route is that nutritional supplementation advice is often included with the results; however, insurance rarely covers such testing, and patients must be aware that the information provided is not designed to replace medical care.
None of these approaches is likely to provide any information regarding the advisability of the two substances with the most evidence supporting their use in glaucoma, namely ginkgo biloba and Mirtogenol. Discuss the potential risks and benefits of these substances with your patients, and provide them with detailed information about how to purchase any product(s) you recommend.
Finally, we can consider genetic and epigenetic factors in our evaluation and management of our patients. Remember that a positive family history of glaucoma is a well-known risk factor for the disease. It may be that the hereditary component in the pathophysiology of glaucoma may be at least partly due to genetic defects that lead to inadequate endogenous antioxidants or vasoregulatory agents. If so, those patients might need relatively higher or lower levels of specific nutrients to compensate for altered function. This is a rapidly emerging area that is not without controversy. Given the wide availability of genetic testing and its interpretation, however, patients are increasingly able to inexpensively access to their own genetic ‘map,’ which is likely to prompt many questions about approaches to minimize risk of disease.
Astute clinicians will remain abreast of developments in this area in order to address these questions responsibly.
Currently, while determining individual nutrient needs and intake levels is possible and arguably strongly advisable, it is not yet standard of care in the optometric practice.
This landscape is rapidly evolving, and many experts predict that testing of this sort will be commonplace as we move toward individualized medicine in all aspects of health care.
Dr. Reed is an associate professor at Nova Southeastern University College of Optometry in Fort Lauderdale, Fla. She teaches and writes extensively about ocular disease, ocular pharmacology and nutrition.
1. Leske MC, Rosenthal J. The epidemiologic aspects of open-angle glaucoma. Am J Epidemiol. 1979;109:250-72.2. Leske MC, Connell AM, Schachat AP, et al. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol. 1995;113:918-24.
3. Quigley HA, Enger C, Katz J, et al. Rsk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994;112:644-9.
4. Tielsch, J. The epidemiology of primary open angle glaucoma. Ophthalmol Clin North Am. 1991;4:649-57.
5. Bengtsson B, Leske MC, Hyman L, et al. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:205-9.
6. Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataracts, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Surv Ophthalmol. 1980;24(suppl):335-610.
7. Armaly MF, Krueger DE, Maunder L, et al. Biostatistical analysis of the collaborative glaucoma study. I. summary report of the risk factors for glaucomatous visual-field defects. Arch Ophthalmol. 1980;98:2163-71.
8. Bengtsson B. The prevalence of glaucoma. Br J Ophthalmol. 1981;65:46-9.
9. Bankes JL, Perkins ES, Tsolakis S, et al. Bedford glaucoma survey. Br Med J. 1968;1:791-6.
10. Hollows FC, Graham PA. Intraocular pressure, glaucoma, and glaucoma suspects in a defined population. Br J Ophthalmol. 1966;50:570-86.
11. Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among black and white Americans. The Baltimore eye Study. Arch Ophthalmol. 1991;91:564-79.
12. Tielsch JM, Katz J, Singh K, et al. A population based evaluation of glaucoma screening: The Baltimore Eye Survey. Am J Epidemiol. 1991;134:1102-10.
13. Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi Study. Ophthalmology. 2004;111:1641-8.
14. Kim JH, Kang SY, Skim NR, et al. Prevalence and characteristics of glaucoma among Korean adults. Korean J Ophthalmol. 2001;25:110-15.
15. Sommer A. Intraocular pressure and glaucoma. Am J Ophthalmol. 1989;107:86-8.
16. Varma R, Wang D, Wu C, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos. Ophthalmology. 2004;111:1439-48.
17. Salinaro T, Cornelius C, Koverech G, et al. Cellular stress response, redox status, and vitagenes in glaucoma: a systemic oxidant disorder linked to Alzheimer’s disease. Front Pharmacol. 2014 Jun 6;5:129.
18. Park C, Kim J. Effect of advanced glycation end products on oxidative stress and senescence of trabecular meshwork cells. Korean J Ophthalmol. 2012 Apr;26(2):123-31.
19. Tezel G, Luo C, Yang X. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci. 2007 Mar;48(3):1201-11.
20. Yanagisawa M, Aida T, Takeda T, et al. Arundic acid attenuates retinal ganglion cell death by increasing glutamate/aspartate transporter expression in a model of normal tension glaucoma. Cell Death Dis. 2015 Mar 19;6:e1693.
21. Iglesias A, Springelkamp H, Ramdas W, et al. Genes, pathways, and animal models in primary open-angle glaucoma. Eye (Lond). 2015 Oct;29(10):1285-98. Epub 2015 Aug 28.
22. Patel S, Mathan J, Vaghefi E, Braakhuis A. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2015 Nov;253(11):1841-50. Epub 2015 Sep 4.
23. Shields MB. Normal tension glaucoma: Is it different from primary open-angle glaucoma? Curr Opin Ophthalmol. 2008;19:85-8.
24. Anderson DR. Glaucoma, capillaries and pericytes: 1. Blood flow regulation. Ophthalmologica. 1996;210:257-62.
25. Leske MC, Su SY, Nemesure B, Hennis A. Incident open-angle glaucoma and blood pressure. Arch Ophthalmol. 2002;120:954-9.
26. Leske MC. Ocular perfusion pressure and glaucoma: Clinical trial and epidemiological findings. Curr Opin Ophthalmol. 2009;20:73-8
27. Leske MC, Heijl A, Hyman L, et al. Early Manifest Glaucoma Trial Group. Predictors of long term progression in the early manifest glaucoma trial. Ophthalmology. 2007;11:1965-72.
28. Leske MC, Wu SY, Hennis A, et al. Risk factors for incident open-angle glaucoma; the Barbados Eye Studies. Ophthalmology. 2008;115(1): 85-93.
29. Siesky B, Harris A, Racette L, et al. Differences in ocular blood flow in glaucoma between patients of African and European descent. J Glaucoma. 2015;24(2):117-21.
30. Ghanem AA, Elewa AM, Arafa LF. Endothelin-1 and nitric oxide levels in patients with glaucoma. Ophthalmic Res. 2011;46:98-102.
31. Mozaffarieh M, Flammer J. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr Opin Pharmacol. 2013 Feb;13(1):43-9.
32. Tezel G, Kass MA, Kolker AE, Wax MB. Comparative optic disc analysis in normal pressure glaucoma, primary opn-angle glaucoma and ocular hypertension. Ophthalmology. 1996;103:2105-13.
33. Ishida K, Yamamoto T, Sugiyama K, Kitazawa. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am J Ophthalmol. 2000;129:707-14.
34. Cavet ME, Vittitow JL, Impagnatiello F, et al. Nitric oxide (NO): an emerging target for the treatment of glaucoma. Invest Ophthalmol Vis Sci. 2014;55(8):5005-15.
35. Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33(3):337-49.
36. Mousa A, Kondkar AA, Al-Obeidan SA, et al. Association of total antioxidants level with glaucoma type and severity. Saudi Med J. 2015 Jun;36(6):671-7.
37. Abu-Amero KK, Kondkar AA, Mousa A, et al. Decreased total antioxidants in patients with primary open angle glaucoma. Curr Eye Res 2013 Sep 38(9):959-64.
38. Park JW, Kwon HJ, Chung WS, et al. Short-term effects of ginkgo biloba extract on peripapillary retinal blood flow in normal tension glaucoma. Korean J Ophthalmol 2011 25:323-8.
39. Ou HC, Lee WJ, Lee IT, et al. Ginkgo biloba extract attenuates oxLDL-induced oxidative functional damages in endothelial cells. J Appl Physiol 2009 106:1674-85.
40. Abu-Ameri KK, Morales J, Bosley TM. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2533-2541.
41. Chen X, Serag E, Sneed K, Clinical herbal interactions with conventional drugs: from molecules to maladies. Curr Med Chem. 2011;18(31):4836-50.
42. Borges G, Degeneve A, Mullen W, Crozier A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J Agric Food Chem. 2010;58(7):3901-9.
43. Yoshida K, Ohguro I, Ohguro H. Black currant anthocyanins normalized abnormal levels of serum concentrations of endothelin-1 in patients with glaucoma. J Ocul Pharmacol Ther. 2013;29(5):480-7.
44. Ohguro H, Ohguro I, Yagi S. Effects of black currant anthocyanins on intraocular pressure in healthy volunteers and patients with glaucoma. J Ocul Pharmacol Ther. 2013;29(1):61-7.
45. Ohguro H, Ohguro I, Katai M, Tanaka S. Two-year randomized, placebo-controlled study of black currant anthocyanins on visual field in glaucoma. Ophthalmologica. 2012;228(1):26-35.
46. Steigerwalt RD, Gianni B, Paolo M, et al. Effects of Mirtogenol on ocular blood flow and intraocular hypertension in asymptomatic subjects. Mol Vis. 2008 Jul;14:1288-92.
47. Steigerwalt RD, Belcaro G, Morazzoni P, et al. Mirtogenol potentiates Latanoprost in lowering intraocular pressure and improves ocular blood flow in asymptomatic patients. Clin Ophthalmol. 2010;4:471-6.
48. Lin SC, Singh K, Lin SC. Association between body levels of trace metals and glaucoma prevalence. JAMA Ophthalmol. 2015. Epub ahead of print.
49. Mozaffarieh M, Fraenkl S, Konieczka K, Flammer J. Targeted preventive measures and advanced approaches in personalised treatment of glaucoma neuropathy. EPMA J. 2010;1(2):229-35.
50. Hermida R, Ayala D, Fernández J, Mojón A. Sleep-time blood pressure: prognostic value and relevance as a therapeutic target for cardiovascular risk reduction. Chronobiol Int. 2013 Mar;30(1-2):68-86. Epub 2012 Oct 25.

