In April 2016, optometry lost a giant when the author of the seminal work Primary Care of the Posterior Segment, Larry Alexander, OD, died. In addition to being an optometric physician, author and educator at the University of Alabama Birmingham School of Optometry, Dr. Alexander was a past president of the Optometric Retina Society (ORS). That group chose to honor his legacy by accepting case reports from optometric residents across the country relating to vitreoretinal disease.
This case, selected by the ORS Awards Committee, was co-winner of the fifth annual Larry Alexander Resident Case Report Contest. The contest is sponsored by Zeiss, Heidelberg and Optos.
Choroidal melanoma is an uncommon malignancy that commonly occurs during mid-life, in both males and females, and can arise from a pre-existent lesion or de-novo.3 Symptoms can range from none to loss of vision or flashes of light.3 There is an increased risk of metastasis with increasing tumor thickness and because of this, the recognition of small choroidal melanoma is critical for both patient safety and survival.4 With the use of multimodal imaging, the visualization of key clinical features and risks associated with choroidal melanoma have been improved and aids in the detection of early malignacy.4 The Collaborative Ocular Melanoma Study (COMS) was an early study performed regarding the standard of care and management techniques for choroidal melanoma.5 COMS reported no benefit from pre-enucleation external-beam radiation as compared to enucleation alone in terms of survival advantage; however, newer innovative treatment and management strategies will be discussed along with this case report.5 Such treatments include plaque radiotherapy and novel techniques currently being researched including AU-011 and DecisionDx (Castle Biosciences Inc.).
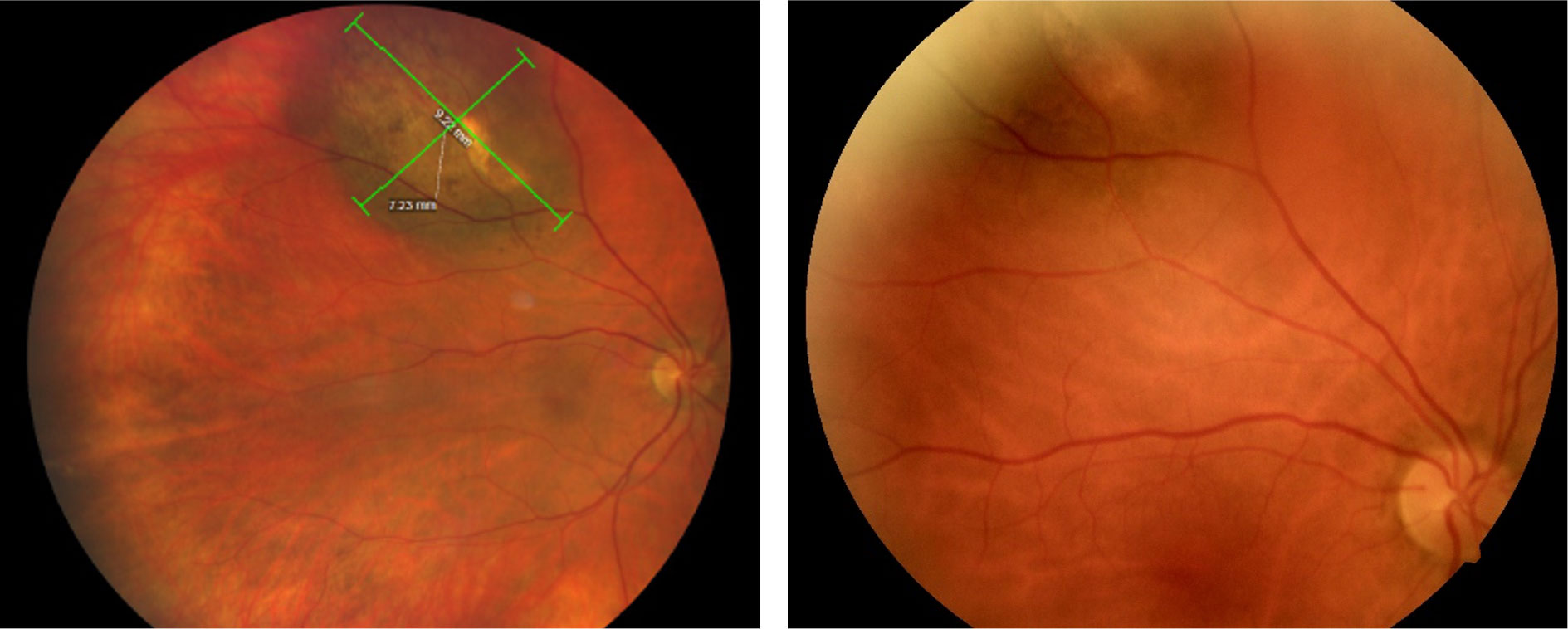 |
| Fig. 1a. (Left) Wide-field color fundus photography of pigmented choroidal tumor of the right eye upon 2020 evaluation. Tumor size measured 9.22mm x.7.23mm. Fig. 1b. (Right) Standard fundus photography taken 16 months prior (2018). Comparison between the two demonstrates obvious expansion of the tumor with the posterior margin now extending past the venous bifurcation and reaching closer to the optic nerve. Click image to enlarge. |
Case Report
A 69-year-old Native American female presented for a general eye exam with a chief complaint of blurred vision with the right eye with the use of her current glasses of three years of age. She admitted that the reduced vision had been long standing and present for over a year. She denied any visual distortions, such as metamorphopsia or scotoma, as well as photopsia. She also reported being denied an updated spectacle prescription at her previous eye exam with another provider. Her medical history was remarkable for arthritis and asthma and ocular history remarkable for a pigmented choroidal tumor of the right eye initially described as a suspicious choroidal nevus three years prior. After close evaluation by a retinal specialist over the next one and a half years, a referral was placed to an ocular oncologist due to the onset of subretinal fluid.
The ocular oncologist extensively educated the patient, noting that the suspicious nevus had a 50% or greater risk of obvious malignant transformation and recommended plaque radiotherapy; however, the patient elected observation. The patient followed up with ocular oncology approximately seven months later at which vision was found to be further decreased to 20/50 in the affected right eye; however, the patient still elected observation. Her family medial history was remarkable for hypertension, diabetes mellitus and cancer, specific to her mother. Her medications included albuterol inhaler as needed for asthma symptoms and hydrocodone taken three times a day. She denied any alcohol or tobacco use and reported being a retired nurse.
At the current visit, her best corrected visual acuities were 20/40 in the right eye without improvement with pinhole and 20/20 in the left eye. Pupil, extraocular motility and confrontation visual field testing were all within normal limits, while her blood pressure was elevated at 168/105mm Hg. Biomicroscopy evaluation of the anterior segment was remarkable for bilateral mild cataracts. The previously noted pigmented choroidal tumor was appreciated in the superior posterior pole with dilated fundoscopy and was then further evaluated with wide-field fundus photography and optical coherence tomography (OCT) imaging. Apart from the choroidal tumor, dilated fundoscopy of the right eye revealed an area of elevation of the inferior macula that extended into the inferior arcade with an absence of a foveal light reflex. Additionally, vitreous syneresis without a posterior vitreous detachment and a healthy optic nerve head with a cup-to-disc ratio of 0.20 were noted. The evaluation of the left eye revealed no pathology, with a healthy macular pigment and foveal contour, vitreous syneresis and optic nerve head with a cup-to-disc ratio of 0.20.
The tumor pigmentation was non-homogenous and the coloration was grayish-green with a hypo-pigmented center and apparent overlying orange lipofuscin deposition. There was a general absence of drusen and no hypopigmented halo was appreciated. The tumor was obviously elevated with fundoscopy and questionable associated subretinal fluid was present. Utilizing the caliber and wide-field colored fundus photography the tumor diameter was measured to be 9.22mm x 7.23mm (Figure 1a). Upon comparison with a fundus photograph (Figure 1b) taken 16 months prior, growth of the lesion was clearly evident since the posterior margin had enlarged past the venous bifurcation, reaching closer to the optic nerve. OCT imaging of the tumor revealed a tumor thickness of 1,382 micrometers with diffuse subretinal hyperreflective pigment deposition causing posterior shadowing within the tumor below (Figure 2).
A small sliver of subretinal fluid was present on the lateral aspects of the tumor with loss of the outer nuclear layer and photoreceptors within the retina overlying the tumor, while the inner retina appeared intact. OCT imaging through the center of the fovea revealed distant subretinal fluid beneath that extended into the inferior arcade and likely accounted for reduced best corrected vision of the right eye (Figure 3). The posterior aspect of the photoreceptors in the fovea had an elongated, fuzzy appearance which was consistent with Dr. Carol Shields’ description of “shaggy photoreceptors”4. The fovea also demonstrated loss of the ellipsoid zone and thinning of the outer nuclear layer. Subretinal fluid accumulation was extensive and reached the inferior arcade as imaged via OCT in Figure 4.
Prior records from ocular oncology seventeen months ago were obtained and reviewed. At that evaluation the ocular oncologist noted the size of the tumor to be 9mm x 6mm x 2.5mm, and the tumor was acoustically hollow via ultrasound. The oncologist also noted trace subretinal fluid confirmed with OCT and orange pigment via autofluorescence, recommending plaque radiotherapy treatment. At the current examination these recommendations were again reiterated to the patient and she was referred back to the ocular oncologist for consideration of radiotherapy. Additionally, she was asked to return to the optometry clinic for re-evaluation in three months; however, she missed both the ocular oncologist and optometry follow up appointments.
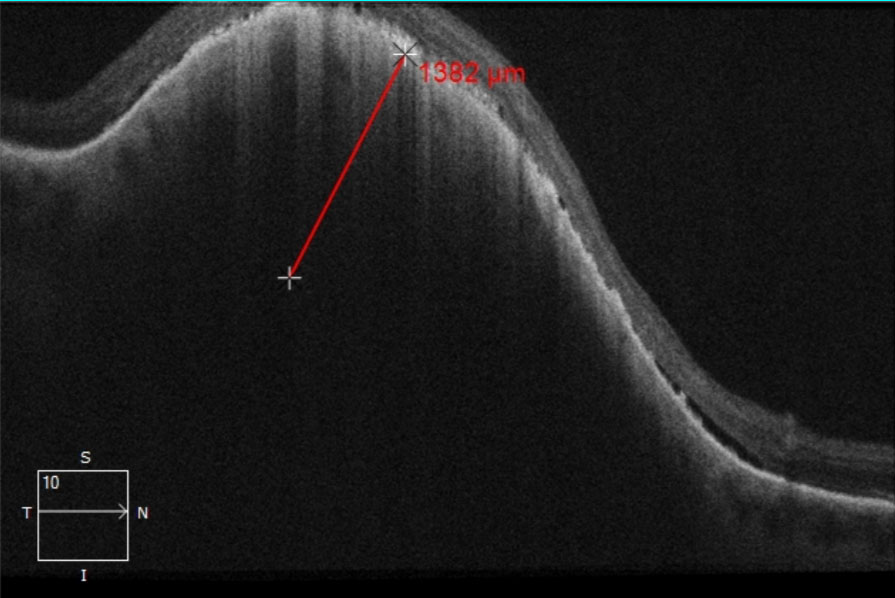 |
| Fig. 2. OCT raster image through the pigmented choroidal tumor. The thickness of the tumor was measured to be approximately 1,382 micrometers. Presence of subretinal fluid was noted along the tumor margins and more evident on the posterior aspect. Additionally, diffuse subretinal hyperreflective pigment deposition causing posterior shadowing within the tumor below was notable and likely signified lipofuscin. Click image to enlarge. |
Differentials
Differential diagnoses considered included choroidal nevus, congenital retinal pigment epithelium hypertrophy (CHRPE), choroidal metastasis and choroidal melanoma. Multimodal imaging can be of great value in distinguishing between these entities and will be discussed in detail.
1. Choroidal Nevus
a. Nevi are benign lesions with feathered, irregular edges usually slate-gray in color.6 Changes to the surface such as overlying drusen and subretinal fluid accumulation can occur over an extended timeframe.6 With the use of enhanced-depth imaging OCT (EDI-OCT), Shields7 described the origination of a choroidal nevus to be within the outer choroid.7 Multimodal imaging often demonstrates that a nevus compresses the choriocapillaris and results in posterior shadowing, both signs frequently present in studied eyes.7 Benign nevi rarely present with orange pigment, substantial thickness, or ultrasonic hollowness, all features present in our patient which suggest malignancy.7
2. CHRPE
a. CHRPE’s present as flat lesions with sharp borders. It is a benign, hyperpigmented lesion within the RPE that typically requires no treatment. Upon imaging, there is typically photoreceptor loss with an overall healthy, normal choroid beneath.7 The patient’s tumor had ill-defined borders, was not very darkly pigmented and had substantial thickness arguing against a CHRPE.
3. Choroidal metastasis
a. Metastatic spread often occurs within the choroid due to its vascular nature usually as a result from lung or breast cancer in men and women, respectively.6 This lesion differs in that it demonstrates a lumpy-bumpy RPE contour via OCT compared to the dome-shaped curvature of a nevus or melanoma.7 Similarly to choroidal melanoma, choriocapillaris compression, subretinal fluid, shaggy photoreceptors and lipofuscin debris can occur.7 Choroidal metastasis coloration typically demonstrates a creamy white pigmentation, standing out from the other differentials.7 Our patient had no known history of systemic cancer and the lesion was pigmented with a smooth anterior surface further arguing against choroidal metastasis as well.
4. Choroidal melanoma
a. Compared to choroidal nevi, small choroidal melanomas are more likely to exhibit shaggy photoreceptors, increased tumor thickness, subretinal fluid and lipofuscin.7 Recognizing these features can aid in the differentiation from nevi which frequently lack these findings.7 Our patient had multiple risk factors for choroidal melanoma that will be further discussed in the next section.
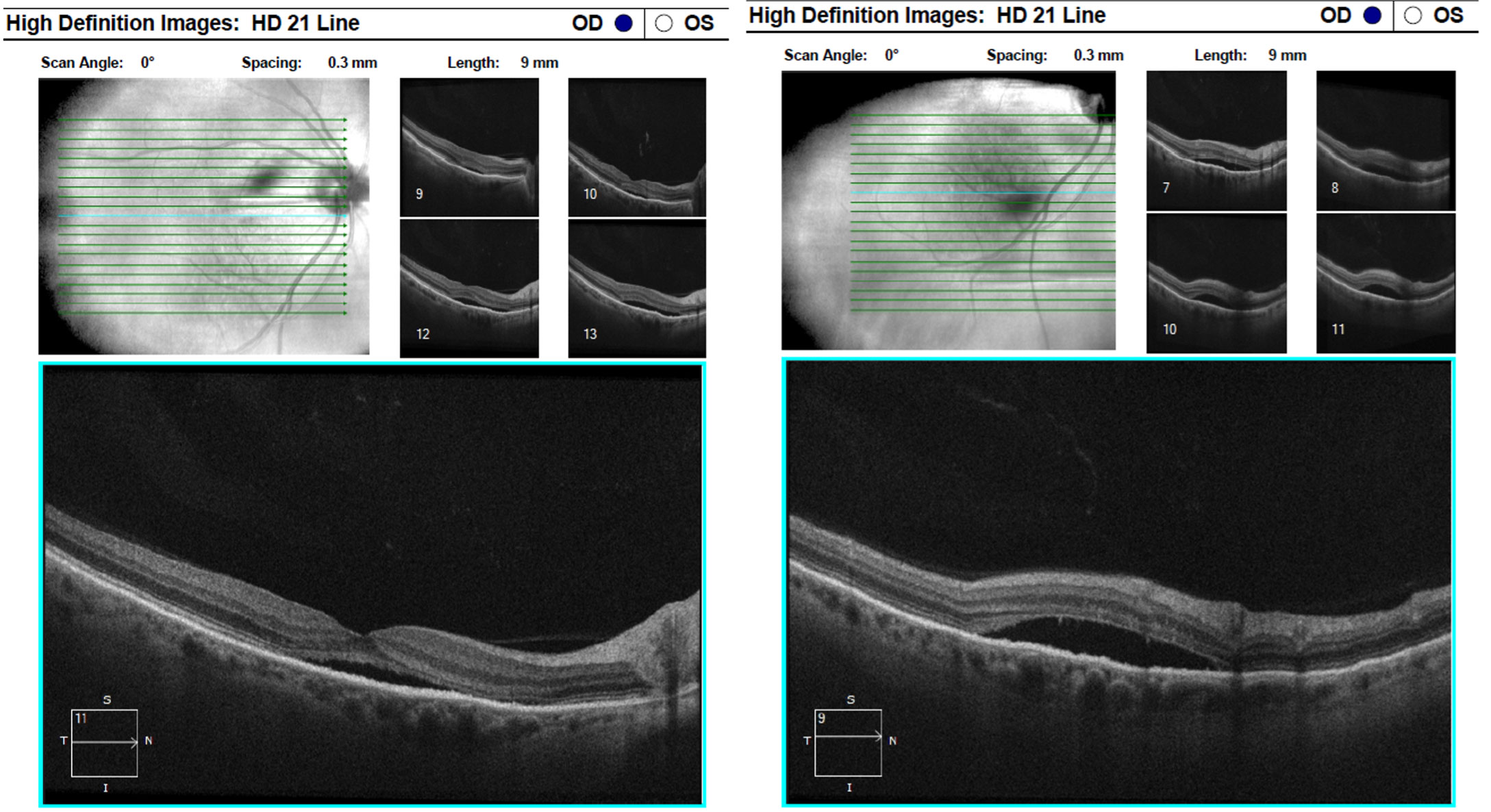 |
| Fig. 3 (Left) and Fig. 4 (Right): OCT raster images through the macula and the inferior arcade demonstrate extensive subretinal fluid with elongated, “shaggy” photoreceptors and outer retinal atrophy. Click image to enlarge. |
Discussion
Choroidal melanoma is the most common, primary intraocular malignant tumor and this location is the second most common site for malignancy within the body.1 Uveal melanomas involve the choroid 90% of the time, while the remaining 10% involve either the ciliary body or iris.1 Commonly affected individuals include fairly-pigmented populations, such as Caucasians of Northern European descent.1 Discovery of a choroidal melanoma typically occurs coincidentally during a general eye exam, with the median age of diagnosis being 55 years, and men and women are equally predisposed.6 There is risk for metastasis, and the most common location is the liver, followed by the lungs and skin.4
Upon fundus examination, a choroidal melanoma appears as an elevated, dome-shaped subretinal mass that commonly takes on a mushroom shape if Bruch’s membrane is breached.1 Tumor pigmentation can range from darkly pigmented to amelanotic, with the latter being more hazardous.1 Similarly, subretinal deposition of orange pigment, lipofuscin, carries greater risk.1 On a more molecular level, a spindle-cell melanoma has a better prognosis than that of an epithelioid melanoma.1 COMS proposed a classification scheme for choroidal melanoma based on apical height via ultrasound and diameter via photography and when utilized for the patient discussed, the pigmented choroidal tumor would be classified as medium in size based on the apical height of 2.5mm via ultrasound performed by the ocular oncologist.8 Additionally, the tumor presented with both darkly pigmented and amelanotic areas, as well as lipofuscin deposition carrying greater risk.
Extensive research has outlined the risk factors for malignant transformation as visualized by multimodal imaging and summarized these in the well-known mnemonic “To Find Small Ocular Melanoma Doing Imaging (TFSOM-DIM).”2 High risk characteristics are as follows: Thickness greater than 2mm on ultrasonography, subretinal fluid confirmed with OCT, symptomatic vision loss (20/50 or less with Snellen visual acuity), orange pigment represented by hyper-autofluorescence on autofluorescence, acoustic hollowness on ultrasonography and tumor diameter greater than 5mm on photography.2
The COMS study similarly noted characteristics associated with a greater risk of growth including greater initial tumor thickness and diameter, presence of lipofuscin, absence of drusen and absence of RPE changes adjacent to the tumor; however, during this study in 1997 multimodal imaging was not yet available.2 Shields et al. estimated the risk for malignant transformation within five years based upon the number of associated risk factors and these are summarized in Table 1.2 Based on TFSOM-DIM and predicted rate of transformation, the patient described in this case is predicted to have a greater than 55% risk by exhibiting all six characteristics.
Enhanced depth imaging describes an OCT imaging method that improves the visualization of ocular structures positioned deep to the retina, specifically the choroid.9 When comparing between choroidal nevi and small choroidal melanoma, the EDI-OCT study performed by Shields et al. showed predictable melanoma characteristics including increased tumor thickness, subretinal fluid, subretinal lipofuscin deposition and RPE atrophy.4 Additionally, multimodal imaging characteristics more commonly associated with melanoma included shaggy photoreceptors, loss of external limiting membrane and inner segment-outer segment junction, irregularity of the inner plexiform and ganglion cell layers and intraretinal edema as compared to nevi.4 The presence of shaggy photoreceptors is considered the hallmark differential between choroidal melanoma and choroidal nevi based on Shields’ study results.4 The appearance results from the photoreceptor cells swelling after being in contact with fresh subretinal fluid. 4 The patient discussed in this case report displayed both shaggy photoreceptors and lipofuscin deposition via OCT as shown in Figures 2-4.
Of note, Shields determined that tumor thickness measurement using EDI-OCT is approximately 54% less when compared with ultrasonography.4 The discrepancy is thought to be the result of ultrasonography including either the overlying retina or sclera along the posterior tumor margin. 4 The commonly known thickness cutoff for suspicion of 2mm refers to ultrasound measurements and cannot be applied to OCT measures, which are expected to be approximately half.4 Therefore, OCT tumor thickness measures of approximately 900 microns or more should be considered suspicious, utilizing the conversion factor from Shields’ research.4 Our patient’s tumor thickness was suspicious by both ultrasonography (2.5mm per ocular oncology) and OCT (1,382 micrometers).
Long-term follow up of the condition is imperative for the safety of the patient to assess potential growth, recurrence, or complications from prior therapy.1 Systemic investigations for metastatic spread is essential in high risk tumors and should include liver ultrasound, liver function tests, chest x-ray and CT/MRI/PET scans.1 Although, due to the lack of effective therapies for metastatic disease, it is not anticipated to have significant improvement in mortality if metastasis is identified.1 The current patient discussed did not obtain medical care through the same electronic medical records system, nor provided updated medical records so we are unaware if any metastasis is present although screening was recommended. However, based on her reported use of Hydrocodone a suspicion of current cancer treatment and likeliness of metastasis can be made due to chronic opioid use to aid with therapy side effects being common.
Primary treatment options for choroidal melanoma include laser photocoagulation, transpupillary thermotherapy (TTT) and plaque radiotherapy.2 The goal of laser photocoagulation is to control tumor growth and minimize vision loss; however, due to post-laser complications such as vitreous hemorrhage the treatment has been abandoned in favor of TTT or plaque radiotherapy.2 TTT encompasses the use of a near IR diode laser to increase the temperature of the tumor resulting in necrosis.2 This method of treatment has been found to to have favorable effects with little collateral damage to surrounding tissues.2 Ultimately, the preferred treatment option for a tumor with three risk factors or more is plaque radiotherapy which is superior to TTT.2 Today, plaque radiotherapy is the most common method of choroidal melanoma management with favorable tumor control at the ten year mark, but is associated with a substantial risk of vision loss.2 A novel therapy currently in clinical trials is the use AU-011, an infrared dye-conjugated virus-like nanoparticle developed for the treatment of small choroidal melanoma.2 Using intravitreal injection of a photosensitizer, the virus-like particle is activated via near IR laser causing cytotoxic effects on the tumor alone.2 Benefits of this innovative method include less collateral damage, less risk of vision loss and the ability of the procedure to be performed in an outpatient setting.2
Due to the potential fatal nature of uveal melanoma, accurate methods to predict metastatic risk are imperative to determine appropriate management strategies to aid in early detection and prevent cancer spread.10 Cook et al.10 developed a gene expression profile (GEP) to improve the prediction of metastasis based on the assessment of the melanoma tumor using real-time polymerase chain reaction gene expression of the biopsied tissue. The tumor is then classified into low or high risk for metastasis in five years.10 A commercially available GEP test is DecisionDx (Castle Biosciences Inc.), has been clinically validated in determining accurate and reliable identification of patients with uveal melanoma with high risk of developing metastases based on the molecular class assignment.10 Cook et al. further report GEP prognostic test proving clinical usefulness as a tool to implement risk-appropriate healthcare decision making based on analytic validity and reproducibility as compared to previous standards.10
Predicted Transformation of Choroidal Nevus into Melanoma after Five Years | |
| No Risk Factor | 1% |
| 1 Risk Factor | 11% |
| 2 Risk Factors | 22% |
| 3 Risk Factors | 34% |
| 4 Risk Factors | 51% |
| 5 Risk Factors | 55% |
Conclusion
Early and accurate detection of choroidal melanoma has greatly improved with the use of contemporary multimodal imaging technology including EDI-OCT, fundus autofluorescence and wide-field fundus photography. Abundant research has identified high risk features for choroidal melanoma and can be easily referenced in a clinical setting using the mnemonic TFSOM-DIM developed by Shields.4 Even following ocular treatment, lifelong systemic monitoring for metastasis is imperative and should include screening of the liver and lungs.4 Contemporary management of choroidal melanoma most often includes plaque radiotherapy and newer, innovative methods are promising. Although uncommon, it is highly critical that eye care providers be familiar with and able to recognize high risk features suggestive of small choroidal melanoma utilizing multimodal imaging.
1. Chan R, Flakner-Radler C, Pettey J, et al. Choroidal melanoma—Europe. AAO. 2014. www.aao.org/topic-detail/choroidal-melanoma-europe 2. Shields C, Lim L, Dalvin L. Small choroidal melanoma: detection with multimodal imaging and management with plaque radiotherapy or AU-011 nanoparticle therapy. Curr Opin Ophthalmol. 2019;30(3):206-14. 3. Wills Eye Hospital. Choroidal melanoma. www.willseye.org/choroidal-melanoma/. Accessed February 15, 2021. 4. Shields C, Kaliki S, Rojanapron D, et al. Enhanced depth imaging optical coherence tomography of small choroidal melanoma. Arch Ophthalmol. 2012;130(7):850-6. 5. Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. ten-year mortality findings and prognostic factors. COMS report number 24. Am J Ophthalmol. 2004;138(6):936-51. 6. Abramson D, Schefler A, Dunkel I, et al. Adult Ophthalmic Oncology: Ocular Diseases. Hollan-Frei Cancer Medicine. 2003; 6. 7. Shields C. EDI-OCT of intraocular tumors. Retina Today. 2013. 8. Margo C. The collaborative ocular melanoma study: an overview. Cancer Control. 2004;11(5):304-9. 9. Shah S, Kaliki S, Shields C, et al. Enhanced depth imaging optical coherence tomography of choroidal nevus in 104 cases. Ophthalmology. 2012;119(5):1066-72. 10. Cook R, Middlebrook B, Wilkinson J, et al. Analytic validity of DecisionDx-Melanoma, a gene expression profile test for determining metastatic risk in melanoma patients. Diagn Pathol. 2018;13(1):13. |

