 A 43-year-old white female presented for a comprehensive eye exam. Her medical history was significant for multiple sclerosis (MS), which was diagnosed five years earlier. Her last MRI was performed more than two years ago.
A 43-year-old white female presented for a comprehensive eye exam. Her medical history was significant for multiple sclerosis (MS), which was diagnosed five years earlier. Her last MRI was performed more than two years ago.
The patient’s pupils and extraocular motilities were normal. Additionally, the dilated fundus examination was unremarkable.
Given her history of MS, we obtained a spectral-domain optical coherence tomography (SD-OCT) scan. The ganglion cell complex analysis revealed mild thinning OU.
Could the changes observed on her ganglion cell analysis be used to determine the status of her MS?
A Connection with the Eye?
MS is an autoimmune disease characterized by nerve demyelination within the central nervous system. Because this process causes reduced axon transmission, patients with MS often experience permanent disabilities.
Traditionally, magnetic resonance imaging has been used to measure the severity of MS. However, it’s often challenging to correlate the clinical signs and symptoms with MRI findings.
Because the retinal nerve fibers are not protected by myelin, clinicians are able to visualize isolated axons via optical coherence tomography. The ability to measure retinal nerve fiber layer integrity effectively provides an indirect method of anterior visual pathway assessment in MS patients.1
Several studies have indicated that patients with MS often exhibit retinal nerve fiber layer thinning when compared to healthy, age-matched patients.1-4 It is important to note that such findings have been documented in MS patients who present either with or without a history of optic neuritis.5 Additionally, subclinical disease manifestation has been linked to decreased macular thickness.6
New Diagnostic Approaches
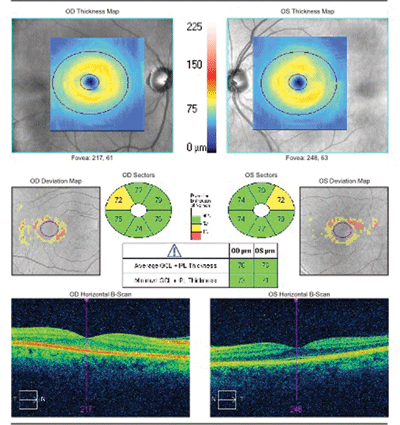
The SD-OCT scan of our 43-year-old patient showed mild ganglion cell/inner plexiform (GCIP) thinning OU.
One study published in January 2013 showed that ganglion cell complex quantification could help determine the extent of MS progression.7
The researchers concluded that acceleration of ganglion cell/inner plexiform (GCIP) thinning was indicative of disease activity and severity.7
All four subtypes of MS were included in this study (isolated syndrome, relapse, and both primary and secondary progressive).
Patients with an established history of optic neuritis––as well as those who manifested the condition during the study––were excluded.
The study population included 164 MS patients and 59 control subjects. All participants underwent a battery of tests over a 21-month duration, including spectral-domain OCT every six months and an annual contrast-enhanced brain MRI.
The researchers determined that subjects who experienced an MS relapse during the study exhibited 42% faster GCIP thinning than those who did not experience a relapse.7
Additionally, MS subjects who manifested new or enhanced lesion growth on MRI experienced 36% to 54% faster GCIP thinning compared to those who did not develop such changes.
Significant inflammation documented early in the disease process secondary to GCIP thinning may be linked to MS-related disability. For example, patients who were diagnosed with MS within the last five years showed a higher annual rate of GCIP thinning than those who were diagnosed with MS more than five years ago.7
This finding suggests an increased likelihood for the patient to exhibit a greater degree of inflammation early in the disease process. Also of interest, the presence of cumulative factors (e.g., the development of new T2 lesions) might increase the rate of GCIP thinning.7
Optical coherence tomography provides clinicians a novel way of measuring axon loss in patients with MS. Retinal nerve fiber layer analysis, macular thickness assessment and ganglion cell complex measurements are instrumental in the diagnosis and evaluation of the condition.
In particular, alterations in ganglion cell density may reflect micro-inflammatory processes in the absence of clinically evident of optic neuritis. Hopefully, in the near future, OCT will enable clinicians to assess the efficacy of prospective neuroprotective agents.
1. Barkhof F, Calabresi PA, Miller DH, et al. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009 May;5(5):256-66.
2. Frohman EM. Modeling axonal degeneration within the anterior visual system: implications for demonstrating neuroprotection in multiple sclerosis. Arch Neurol. 2008 Jan;65(1):26-35.
3. Sergott RC, Frohman E, Glanzman R, et al. The role of optical coherence tomography in multiple sclerosis: expert panel consensus. J Neurol Sci. 2007 Dec 15;263(1-2):3-14.
4. Trip SA, Schlottmann PG, Jones SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005 Sep;58(3):383-91.
5. Oberwahrenbrock T, Schippling S. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int. 2012;2012:530305.
6. Burkholder BM, Osborne B. Macular volume determined by optical coherence tomography as a measure of neuronal loss in multiple sclerosis. Arch Neurol. 2009 Nov;66:1366-72.
7. Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013 Jan 1;80(1):47-54.

