 |
A new patient, a 69-year-old black male, presented to the office for continuation of care in 2013. He suffered a self-reported “stroke” in his right eye approximately five years earlier that had affected his vision somewhat, and his ocular history was also significant for cataracts in both eyes. At that initial visit, his best-corrected visual acuities (BCVA) were 20/50 OD and 20/25 OS through hyperopic astigmatic correction. Medications included antihypertensives, antihypercholesterolemics and NSAIDs.
His initial visit was to establish care and to see if there was anything that could be done to improve his vision in the right eye, though he said he was previously told the changes to the right eye were permanent.
His pupillary responses indicated a +1 afferent pupillary defect (APD) OD. He had symmetric early nuclear cataracts, consistent with the BCVA in his left eye.
The initial evaluation of his optic nerves demonstrated a couple of significant findings. The right optic nerve was somewhat diffusely pale, with a somewhat thinned neuroretinal rim and a cup-to-disc estimated at 0.45 x 0.6. The neuroretinal rim in the left eye was equally thinned, but was plush and well perfused on funduscopy. The retinal vasculature in his right eye was slightly attenuated, especially along the course of the superior temporal artery; overall arterial attenuation was noted in the right eye more so than in the left. Peripheral retinal evaluations were unremarkable.
Given the findings, I suspected that he had had an ischemic event of either an embolic nature or local infarction of his optic nerve in the right eye in previous years, leaving him with decreased BCVA in the left eye, and I could offer no significant help in improving his vision.
However, his optic nerves, aside from the pallor in the right, were suspect for the possibility of glaucoma. Accordingly, he was scheduled for testing to obtain a baseline of optic nerve functioning, including threshold visual fields, optical coherence tomography (OCT) and Heidelberg Retina Tomograph (HRT 3) scanning, as well as multimodal imaging of the posterior segment.
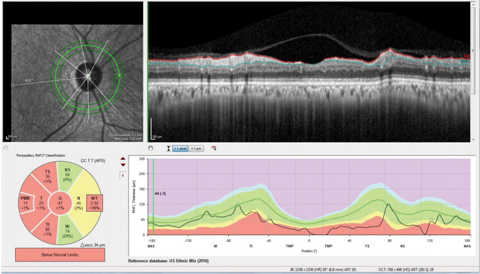 |
| Fig. 1. The neuro protocol retinal nerve fiber layer circle scan with the papillomacular bundle (PMB) represented in the center of the double hump plotting we are used to viewing, as opposed to the standard RNFL circle scan which has the papillomacular bundle at the ends of the double hump plot. Note also the segmentation of the papillomacular bundle in the Garway-Heath sector analysis in this patient with non-glaucomatous optic neuropathy. Click image to enlarge. |
A Closer Look
He complied with these requests, and not surprisingly, his right eye was found to have an altitudinal visual field defect inferiorly involving fixation, as well as a questionable field defect in the superior arcuate area, though visual field reliability indices were not the most accurate. His visual field study in the left eye was essentially normal. Structural imaging of the optic nerves with both HRT 3 and OCT demonstrated a slightly thinned neuroretinal rim consistent with the initial clinical findings. Macular scans demonstrated superior hemispheric thinning of the total retinal thickness in the right eye, consistent with a regional infarction.
However, in the context of glaucoma, I could attribute no direct findings to uncontrolled glaucoma in either eye at the same time; so, I elected to proceed with simple monitoring of the optic nerves in the context of glaucoma and a suspected ischemic event, as well as his cataracts.
Following Through
Over the next couple of years, being seen twice a year, his cataracts progressed slightly and visual acuity decreased correspondingly. However, in mid 2016, when he presented for his scheduled follow up visit, he complained of worsening vision in his right eye. At that time, visual acuity in the right eye had dropped to 20/200 and maintained an APD. On dilated fundus evaluation, his cataracts had progressed in both eyes, lightly more in the right than in the left, but not to the level matching his BCVA in the right eye.
Medications at this visit included Cozaar (losartan potassium, Merck) Dyazide (hydrochlorothiazide/triamterene, Novartis), Zocor (simvastatin, Merck) and 81mg aspirin, with no reported allergies to medications. While the cataract had worsened in the right eye more than the left, so did the pallor of the right optic nerve as compared with previous visits. His macular and vascular evaluations were stable from baseline visits.
Given that both progression of cataracts and a suspected reoccurrence of the ischemic event in the right eye were contributing to decreased vision in the right eye, we looked at possible etiologies of progressive ischemic events that appeared concurrent with cataract progression, before heading to cataract surgery.
Differential diagnoses in this particular case included a reoccurrence of the ischemic optic neuropathy, as well as carotid and ophthalmic artery insufficiency. While no retinal emboli were noted, nor was there any history of acute ischemic neurological events, his initial vascular picture in the right eye was consistent with the possibility of retinal vascular insufficiency rather than an acute ischemic optic neuropathy.
Lab testing including erythrocyte sedimentation rate and high-sensitivity C-reactive protein were normal, as were carotid doppler studies. Referral to the patient’s primary care provider was made to facilitate tight blood pressure and cholesterol control, to ascertain other possible concurrent vasculopathies and to ultimately gain clearance for cataract surgery. Following an exhaustive work up, the patient was cleared for cataract surgery, which was performed in 2016.
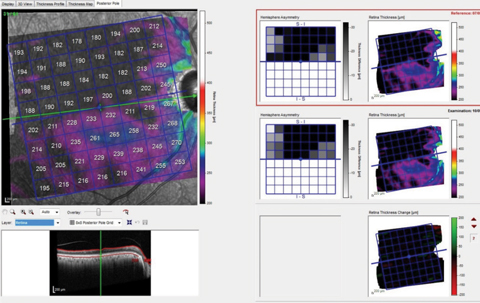 |
| Fig. 2. These images show the patient’s total macular retinal thickness over two separate visits, with essentially no discernible change over the (short) time frame between scans. Click image to enlarge. |
Discussion
The old adage that patients are entitled to as many diseases as they choose certainly is evident here. It is our job to sift through the many possible causes of decreased vision, and to render the appropriate care. You will come across many patients, similar to the one discussed here, who have either frank glaucoma or are a glaucoma suspect and, concurrently, have some other disease process or processes that can confound your evaluation.
In this case, we sought the underlying cause of the decreased vision in the right eye beyond the obvious progression of the cataract. While no specific identifiable etiology was identified from the preoperative studies, it does appear as though the patient had a reflare of his nonglaucomatous optic neuropathy, as evidenced by both the increase in optic disc pallor as well as the postoperative BCVA in the right eye of 20/80-.
Pallor of the neuroretinal rim is not consistent with glaucoma, but is consistent with the nonglaucomatous optic neuropathies. Therefore, if you suspect that a patient has glaucoma, and you also see concurrent neuroretinal rim pallor, there most likely is another nonglaucomatous process occurring.
And then the question, and subsequent management, hinges upon continued monitoring. How do you manage such patients? How do you evaluate these patients? The answer is quite simple. You follow these patients just as you would any other glaucoma patient, with a specific emphasis on the hallmark of glaucoma progression: change over time.
Know Your Imaging
How you image these patients is critical. For example, the standard retinal nerve fiber layer (RNFL) circle scan may be of limited value in these patients, as the RNFL perioptic circle scans look at areas adjacent to, but not at, the optic nerve. Also, since glaucoma structurally involves the ganglion cells, approximately 50% of which come from the macula, macular scans can be helpful. Keep in mind, total macular thickness scans can be confounded by diseases such as age-related macular degeneration, macular puckering or vitreomacular traction.
Knowing the limitations of your imaging equipment of choice is critical in selecting the most appropriate techniques. For example, consider a neurological protocol RNFL circle scan (Figure 1). This scan is different from the normal RNFL circle scan in two important ways. The first is that the typical RNFL circle scan begins temporally and ends temporally, essentially starting and stopping in the papillomacular bundle. The neuro RNFL circle scan, on the other hand, begins nasally and ends nasally, thereby scanning through the entire papillomacular bundle in one sweep. Secondly, reference database comparison with the normal papillomacular bundle parameters are available with this scan, whereas with normal RNFL circle scans the papillomacular bundle is not segmented out.
In the case presented, the initial evaluation demonstrated thinning and loss of retinal thickness in the right eye, especially in the superior hemisphere. On the contrary, ganglion cell layer loss is consistent with glaucoma, and not consistent solely with ischemic events. While looking for change over time, note in Figure 2 the relative stability of overall retinal thickness (as well as how dramatically thinned the entire retina is, especially in the superior hemisphere). This implies that the vascular compromise is stable, but does not reliably comment on stability of the ganglion cell layer.
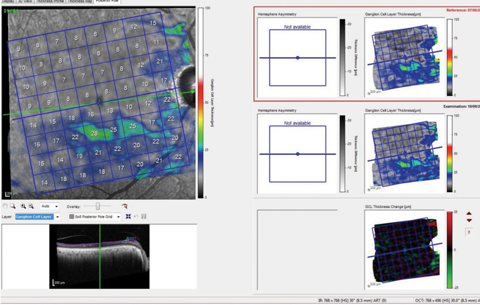
Additional imaging shows the segmented macular ganglion cell layer of the same patient’s right eye (Figure 3). Since this is showing only the ganglion cell layer, it is not confounded as much by other macular diseases, making evaluation of the situation from a glaucoma perspective much easier. However, the ganglion cell layer change analysis of the left eye also seems to indicate some change, which would be attributable to conversion to glaucoma.
 |
| Fig. 2. These images show the patient’s total macular retinal thickness over two separate visits, with essentially no discernible change over the (short) time frame between scans. Click image to enlarge. |
Focus on Structure
A portion of our patient’s vision in the right eye did decrease because of the cataract, which was subsequently addressed, resulting in improved BCVA, but the BCVA was still not as good as it initially was when the patient was first seen, due to the reflare of the ischemic optic neuropathy. If you’ve noticed, I have not mentioned the patient’s intraocular pressures (IOP). The crux of this case is the structural issue occurring in the right eye due to both glaucomatous and nonglaucomatous optic neuropathies.
Since IOP is our only modifiable risk factor, the starting point is somewhat irrelevant (assuming it is not ‘too high’; therefore, a reduction in IOP may be of some benefit for his right eye). Initial IOPs have averaged 12mm Hg to 14mm Hg OU, with average central corneal thicknesses.
So should we lower IOP by a couple of points in the right eye, to mitigate any damage from glaucoma? It can certainly be argued yes. How much? Time will tell.
The key to determining whether we made the right move is answering the root question in the management of all glaucoma patients: What is changing over time?

