 |
A 13-year-old female was referred for reduced vision (20/40) in her left eye with a concurrent abnormal screening visual field, reportedly elevated intraocular pressure (IOP), and an afferent pupillary defect (APD). Her exam was three weeks earlier and she was previously referred to an ophthalmologist more than a year earlier, but her mother did not understand why and did not take her.
When presented with painless vision loss in a young patient with these findings, there are numerous diagnostic possibilities.
The key piece of diagnostic information was her IOP: she measured 28mm Hg OD and 43mm Hg OS by Goldmann applanation. Her pachymetry was slightly thick at 593µm OD and 595µm OS, but these values will not significantly impact an IOP of 43mm Hg. There were no biomicroscopic or gonioscopic abnormalities and both angles were open. The reason for the pronounced visual field loss, reduced vision and left APD was asymmetric glaucomatous damage. She was diagnosed with juvenile open angle glaucoma (JOAG).
Congenital and pediatric glaucoma represent a family of conditions that present, either in primary or secondary form, from birth to age 18. Congenital glaucoma is mainly addressed surgically while other pediatric forms are case dependent and may be treated either medically or surgically.1 In such cases, knowing which medications are effective and safe is invaluable. Unfortunately, very little information is available on the pediatric safety of topical glaucoma medications. For most glaucoma drugs, pediatric use is labeled as “not recommended.”2
Here, we review what the evidence-based literature tells about the use of the most common glaucoma medications in children.
Beta-blockers
Though these were the cornerstone of management for adult primary open angle glaucoma for many years, they must be applied judiciously, even for adults, due to the systemic risks associated with them.
Many clinicians avoid them completely in children. However, the literature shows topical beta blockers can be an effective and safe therapy when used in children.3-9 One report saw 45% of children using topical timolol demonstrate a 10mm Hg reduction in IOP.3 Another study noted that, while pediatric patients experienced approximately a 25% reduction in IOP from timolol use, no patient was controlled on this medication alone. Adverse effects in this report were low, though 7% of patients required timolol cessation.4
In a study involving 50 pediatric patients, only 4% developed systemic side effects.5 In another report that looked at 34 children with glaucoma, the addition of timolol to maximally tolerated medical therapy saw a definite improvement in 10 patients, modest or equivocal improvement in 11, and no substantial benefit in 13.6 In that same study, children older than five showed an average reduction in resting pulse rate of 6 BPM, but those under five years of age demonstrated no change in the resting pulse rate.6 Only one patient was discontinued from timolol for a possible adverse effect.6
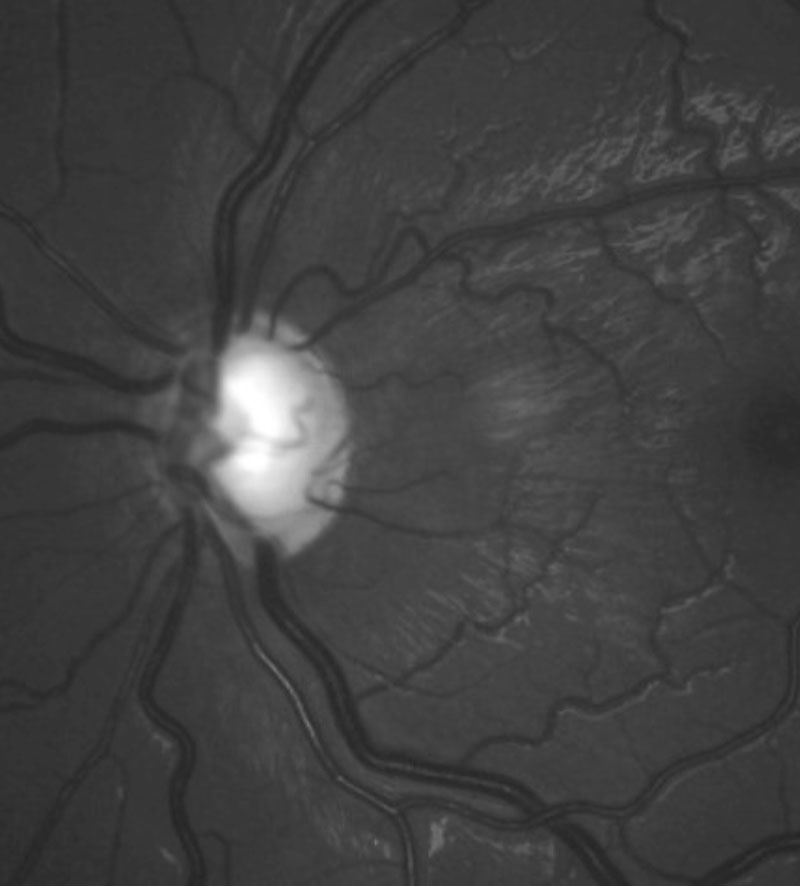 |
| The left glaucomatous optic disc of the patient. |
Prostaglandin Analogs
These have become a favorite among practitioners due to superior efficacy and an excellent safety profile. However, they have not demonstrated similar efficacy in children.10
A 1999 study of 31 children with glaucoma saw an average IOP reduction of only 0.2mm Hg. Eighty percent of the children were non-responders (defined as experiencing a less than 15% IOP reduction with latanoprost). Of the 20% that did show response to latanoprost, those children were of an older cohort and tended to have juvenile open-angle glaucoma. In this study, latanoprost was well-tolerated both ocularly and systemically by all children.11 A 2004 report shows greater IOP reduction, but their cohort was older (12 to 18 years old).12
In a report on pediatric glaucoma associated with port-wine stain, investigators noted IOP control was achieved in only 50% of cases treated with latanoprost.13 Another research team noted that latanoprost is effective in only a minority of pediatric glaucoma cases.14 In contrast, a 2017 report found travoprost to be non-inferior to timolol in lowering IOP in patients with pediatric glaucoma or ocular hypertension.15 Travoprost was well-tolerated, and no treatment-related systemic adverse events were reported.15 Prostaglandin analogs are safe and well tolerated, but unfortunately, not particularly effective in the pediatric glaucoma population. Older children who have juvenile-onset open angle glaucoma see the most benefit.
Alpha-2 Adrenergic Agonists
Research demonstrates that the efficacy of brimonidine in children is approximately 5mm Hg reduction in IOP from baseline when used as a primary or adjunctive agent.16,17 However, brimonidine, a selective alpha-2 agonist, crosses the blood-brain barrier and potentially affects the central nervous system (CNS).18
This medication has an unacceptable level of adverse events in children.16-21 Local adverse reactions such as stinging and burning are common.15 The overall adverse event rate for children using brimonidine was 84% in another report.16 However, CNS effects of brimonidine are potentially more serious; the most notable of these in children has been lethargy and somnolence.16-21 The reported rate of somnolence has ranged from 17% to as much as 76%.16-18 One report noted that brimonidine induced fainting in two children.20 Another reported on coma being induced.21
Despite the efficacy of brimonidine, the side effect profile in children appears unacceptable, especially for those under age eight.
Carbonic Anhydrase Inhibitors
Modestly effective in adults, this class has had little use in the pediatric population. However, studies do demonstrate surprising results with good efficacy and tolerability of both oral and topical carbonic anhydrase inhibitors (CAIs).22-25 One found that topical dorzolamide did not work as well as oral acetazolamide, but did note a significant IOP reduction in the pediatric group, with dorzolamide being well tolerated.24 Another noted a similar efficacy between dorzolamide and acetazolamide without any adverse effects and concluded that topical CAIs can potentially replace oral CAIs to manage pediatric glaucoma.23
Topical dorzolamide appears tolerable, with a local adverse reaction rate of 3% in one report, and an IOP reduction of up to 23% in children younger than six.22 It seems that topical CAIs may be an unnecessarily overlooked class of medications for use in the management of pediatric glaucoma.26
Pressure Relief
Based upon literature reports and personal experience, a topical CAI—and, if necessary, a beta-blocker—seem to work well for children in need of medical pressure reduction. A prostaglandin analog may work if the child is older. Brimonidine should be avoided in children, especially those younger than eight years.
In the young girl presented here, prostaglandin analogs predictably had no effect. She eventually ended up using Cosopt (dorzolamide/timolol fixed combination, Merck), which lowered her IOP to a range of 15mm Hg to 17mm Hg in each eye.
While it may not be common to see children with glaucoma in a primary care practice, realize that the condition does exist, and patients may seek your expertise. Knowing which medications are safe and beneficial is a crucial aspect of managing these youngsters.
1. Tanimoto S, Brandt J. Options in pediatric glaucoma after angle surgery has failed. Curr Opin Ophthalmol. 2006;17(2):132-7. 2. Chung I, Buhr V. Topical ophthalmic drugs and the pediatric patient. Optometry. 2000;71(8):511-8. 3. Hoskins HD Jr, Hetherington J Jr, Magee SD, et al. Clinical experience with timolol in childhood glaucoma. Arch Ophthalmol. 1985;103(8):1163-5. 4. McMahon CD, Hetherington J Jr, Hoskins HD Jr, et al. Timolol and pediatric glaucomas. Ophthalmology. 1981;88(3):249-52. 5. Zimmerman TJ, Kooner KS, Morgan KS. Safety and efficacy of timolol in pediatric glaucoma. Surv Ophthalmol. 1983;28 Suppl:262-4. 6. Boger WP 3rd, Walton DS. Timolol in uncontrolled childhood glaucomas. Ophthalmology. 1981;88(3):253-8. 7. Talbot AW, Russell-Eggitt I. Pharmaceutical management of the childhood glaucomas. Expert Opin Pharmacother. 2000;1(4):697-711. 8. van Emelen C, Goethals M, Dralands L, et al. Treatment of glaucoma in children with Sturge-Weber syndrome. J Pediatr Ophthalmol Strabismus. 2000;37(1):29-34. 9. Koraszewska-Matuszewska B. Pharmacotherapy of congenital glaucoma in young children. Klin Oczna. 1999;101(5):393-6. 10. Enyedi LB, Freedman SF.Latanoprost for the treatment of pediatric glaucoma. Surv Ophthalmol. 2002;47 Suppl 1:S129-32. 11. Enyedi LB, Freedman SF, Buckley EG. The effectiveness of latanoprost for the treatment of pediatric glaucoma. J AAPOS. 1999;3(1):33-9. 12. Urban B, Bakunowicz-Lazarczyk A, Mrugacz M, et al. The effectiveness of latanoprost for the treatment of pediatric glaucoma. Klin Oczna. 2004;106(1-2 Suppl):243-4. 13. Ong T, Chia A, Nischal KK. Latanoprost in port wine stain related paediatric glaucoma. Br J Ophthalmol. 2003;87(9):1091-3. 14. Ravinet E, Mermoud A, Brignoli R. Four years later: a clinical update on latanoprost. Eur J Ophthalmol. 2003;13(2):162-75. 15. Dixon ER, Landry T, Venkataraman S, et al. A 3-month safety and efficacy study of travoprost 0.004% ophthalmic solution compared with timolol in pediatric patients with glaucoma or ocular hypertension. J AAPOS. 2017;21(5):370-374. 16. Montero-de-Espinosa I, Morales C, Marquez-de-Aracena R. Ocular hypertension in children treated with brimonidine 0.2%. A clinical study. Arch Soc Esp Oftalmol. 2006;81(3):155-60. 17. Al-Shahwan S, Al-Torbak AA, Turkmani S, et al. Side-effect profile of brimonidine tartrate in children. Ophthalmology. 2005;112(12):2143. 18. Enyedi LB, Freedman SF. Safety and efficacy of brimonidine in children with glaucoma. J AAPOS. 2001;5(5):281-4. 19. Levy Y, Zadok D.Systemic side effects of ophthalmic drops. Clin Pediatr (Phila). 2004;43(1):99-101. 20. Bowman RJ, Cope J, Nischal KK. Ocular and systemic side effects of brimonidine 0.2% eye drops (Alphagan) in children. Eye. 2004;18(1):24-6. 21. Berlin RJ, Lee UT, Samples JR, et al. Ophthalmic drops causing coma in an infant. J Pediatr. 2001;138(3):441-3. 22. Ott EZ, Mills MD, Arango S, et al. A randomized trial assessing dorzolamide in patients with glaucoma who are younger than 6 years. Arch Ophthalmol. 2005;123(9):1177-86. 23. Rehurek J, Vancurova J. Trusopt, a local carboanhydrase inhibitor, in the treatment of glaucoma in children (preliminary report). Cesk Slov Oftalmol. 1998;54(2):82-5. 24. Portellos M, Buckley EG, Freedman SF. Topical versus oral carbonic anhydrase inhibitor therapy for pediatric glaucoma. J AAPOS. 1998;2(1):43-7. 25. Rehurek J, Spicarova R, Vancurova J. Effect of Trusopt on normal intraocular pressure values in children. Cesk Slov Oftalmol. 2000;56(6):366-9. 26. Coppens G, Stalmans I, Zeyen T, Casteels I. The safety and efficacy of glaucoma medication in the pediatric population. J Pediatr Ophthalmol Strabismus. 2009;46(1):12-8. |

