Is it time for us to rethink our dry eye diagnostic and treatment philosophy? In the traditional approach to dry eye disease (DED), the patient’s subjective symptoms determine the timing and course of management: treatment starts when the problem is brought to the practitioner’s attention. Patient reports of burning, foreign body sensation and daily use of artificial tears often lead to the diagnosis after patients try numerous drops with minimal to no long-term relief. They come to us for better treatment options.
Unfortunately, the first treatment that patients are often given is another artificial tear, which may be effective for mild or episodic dry eye, but addresses none of the underlying pathophysiology. If no improvement is seen, anti-inflammatories, nutraceuticals, antibiotics and punctal occlusion are all options, depending on severity, as recommended by the International Task Force on Dysfunctional Tear Syndrome and the 2007 Dry Eye Workshop.1,2
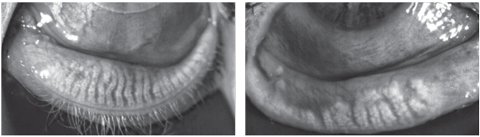 |
| At left: Relatively healthy meibomian glands with piano-key like linear glands running the length of the eyelid. At right: Severe MG drop-out and dilation of the ductal tissues. Click image to enlarge. |
Though many patients can experience symptomatic improvement with this approach, the root cause of the condition may not be identified and treated. Let’s take another approach to dry eye by considering the role of the meibomian glands in DED and recognizing them as the pivotal factors in its development and long-term prognosis.
According to the International Workshop on Meibomian Gland Dysfunction (MGD), the condition is a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction, and qualitative and quantitative changes in the glandular secretion. Meibomian gland dysfunction may result in alterations of the tear film, symptoms of eye irritation, clinically apparent inflammation and ocular surface disease. Additionally, meibomian gland dysfunction may well be the leading cause of DED globally.3 If this is the case, it demonstrates the importance of addressing the meibomian glands and evaluating all our patients—both the asymptomatic as well as the symptomatic. Here are two things to consider:
(1) If we can restore and optimize meibomian gland function, will our intervention halt the progression of the disease?
(2) Can we promote ocular surface wellness for all our patients? To do this, we need to evaluate every ocular surface disease patient for meibomian gland dysfunction and identify the condition at the earliest stages. The inflammatory component of DED may be the cause, or a result of meibomian gland dysfunction, and use of the appropriate anti-inflammatory therapies may be needed as adjunctive therapy.
Let’s Talk Meibomian Gland Structure
Let’s talk briefly about their anatomy. There are approximately 31 glands in the upper lid and approximately 26 glands in the lower lid, with the upper being roughly 5.5mm in length and the lower being near 2mm in length. All glands are spread vertically throughout the tarsal plates in both the superior and inferior lids. Anterior to the tarsal plates lies the orbicularis oculi muscle, which assists in milking the glands during a blink. Each individual gland contains 10 to 15 acini filled with secretory cells responsible for the production of the meibum, which forms the lipid layer of the tear film upon expression from the glands. The acini in every gland cluster around and empty contents into a long central duct from which the meibum is delivered. Surrounding the terminal part of every gland is Riolan’s muscle. During a blink, this muscle—along with the orbicularis oculi—contracts and assists with the delivery of meibum out of the duct and onto the lid margin. This exit point can be described as an orifice.4
 |
| All glands are spread vertically throughout the tarsal plates in both the superior and inferior lids. |
As a sebaceous gland, the meibomian gland produces meibum via holocrine secretion. The contents of the oily meibum include wax and sterol esters (comprising approximately 77%), including fatty acids, fatty alcohols and cholesterol, phospholipids (8%), and digylcerides, triglycerides and hydrocarbons (9%).5 Meibocytes located in acini are secretory cells responsible for the production of meibum. During maturation, the meibocytes’ nuclei shrink and disintegrate, forming the oily product. Meibocyte production is constant, which accounts for the continual secretion of oil.
It is important to differentiate between secretion and delivery of meibum—secretion refers to oil production and delivery refers to its expulsion out of the gland orifice. Meibum, though constantly secreted, is only delivered to the lid surface during a blink. If blockage of the orifice occurs, meibum builds up and the gland eventually atrophies.4 Active delivery of meibum occurs in 45% of glands at any given time and decreases by 50% between the ages of 20 and 80 years, which may be due to gland atrophy.7
While the anatomy of the meibomian glands and the eyelids contains much greater complexity than described here, the importance of evaluating the meibomian gland structure cannot be emphasized enough. Make sure to assess the dry eye patient or suspect for these two structural deficits of the meibomian glands: (1) gland dropout and (2) duct dilatation. Both findings indicate chronic meibomian gland dysfunction and reduced gland function, and can be staged by severity scale. Several commercially available products can assess the structure of the meibomian glands.
What’s the Function?
By understanding the structure of the meibomian glands, both doctors and patients are better able to distinguish normal vs. abnormal gland function. As we know, the meibomian glands secrete the meibum responsible for the formation of the tear film’s outer layer. The meibum aids in reducing the evaporation of tears from the front surface of the eye, increasing the surface tension and forming an optically superior tear meniscus.
The historical idea of three distinct and independent layers of the tear film—the mucin, aqueous and lipid layers—is not quite accurate. More likely, each of the three layers interacts and blends heavily with each other. The proper proportion of each of these three layers is likely as important to ocular surface disease as a deficiency in just one. Disrupting the balance of any of these layers will lead to a dysfunctional tear film, which is not able to protect and nourish the surrounding tissues properly, resulting in inflammation of the ocular surface.
| Lid notching from drop out. |
Any impact on the function of the meibomian glands initiates a domino effect of dysfunction that ultimately contributes to dry eye signs and symptoms. Hyperkeratinization—the leading cause of MGD—in turn causes obstruction of the gland orifice, resulting in meibum build-up and subsequent dilatation of the gland, loss of secretory meibocytes resulting in acini and gland atrophy, and decreased meibum secretion.4 Multiple etiologies may be at the root of hyperkeratinization: aging, hormonal changes, medication and chemical toxic effects, products of meibomian lipid breakdown and external factors such as epinephrine eye drops and contact lens wear are all potential culprits.4 These factors, in addition to blink inhibition, contribute to an increase in evaporative stress that leads to hyperkeratinization and meibomian gland dysfunction.8
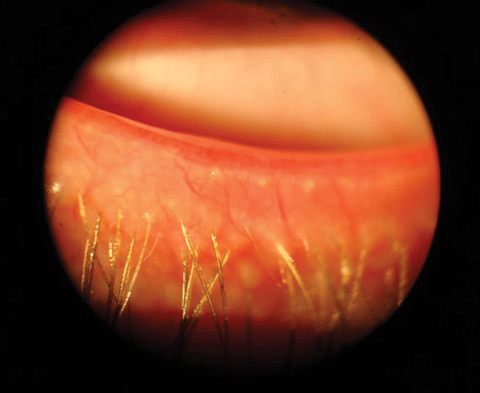 |
| Capped meibomian gland orifices. |
Here are some of the potential hyperkeratinization and meibomian gland dysfunction drivers:
• Omega-3 fatty acids. Some have theorized that a component of meibomian gland dysfunction is related to decreased lipid levels in the meibum. However, a study evaluating the benefit of dietary omega-3 fatty acid (FA) supplementation on dry eye signs and symptoms in patients with meibomian gland dysfunction found no increased omega-3 levels in the meibum after a year of daily supplementation with approximately 3.5g of omega-3s.5 This is not to say that no benefit to omega-3 FA supplementation exists. However, the benefit of supplementation lies in the reduction of inflammation, not in reducing an omega-3 deficit within the meibum.5
• Sex steroids. It is evident that the sex steroids—androgens, estrogens and progestanes—influence the function of sebaceous glands (which include the meibomian glands) throughout the body. Androgens increase lipid production in the meibomian glands and decrease genetic activity responsible for hyperkeratinization. Estrogens, on the other hand, decrease the activity of sebaceous glands. In meibomian glands specifically, this decrease in activity is seen with lipid and fatty acid catabolism and suppression of lipid production genes. The effect of progestanes on meibomian gland function is unclear. Research suggests that progestanes in females are analogous to testosterone in males with respect to its effect on meibomian gland function. Overall, the consensus is that sex steroids affect the meibomian glands, which can explain the sex-dependent symptomatic differences in patients, as well as provide an explanation for the fact that certain disease states create a greater number of dry eye signs and symptoms.4,5
• Glaucoma considerations. Many studies have looked at the correlation between glaucoma medication usage and meibomian gland dysfunction. Most glaucoma drops contain the preservative benzalkonium chloride (BAK). Due to the chronic nature of glaucoma, patients are expected to be on drops long term. Research shows an increase in epithelial holes, a loss of peripheral microvilli and corneal surface cells wrinkling following administration of BAK. According to researchers, BAK also lowers goblet cell density in the conjunctival epithelium.9 All of this sets the stage for a chronic, subclinical, inflammatory environment, which contributes to meibomian gland dysfunction.
• Bacteria and lid flora. A study that evaluated bacteria cultured from eyelids found that these organisms are able to break down healthy meibum, releasing free fatty acids, which irritate the epithelium, triggering an inflammatory cascade. Research shows patients with blepharitis have increased levels of phospholipase A2, an enzyme that catalyzes phospholipids, leading to production of inflammatory mediators such as prostaglandin and leukotriene.10 This secondarily contributes to hyperkeratinization of the meibomian glands as well as changes in the lipid profile of the meibum.11 Although a certain amount of bacteria present on the eyelids is normal, overgrowth may lead to hyperkeratinization and change in the meibum lipid profile.
• Contact lens wear. Contact lenses have been found to contribute to obstructive meibomian gland dysfunction and symptoms of dry eye disease. The exact pathophysiology behind it remains unknown. Researchers have found contact lens patients in their 30s with gland dropout equal to that of a normal population in their 80s; the finding is independent of contact lens type.12
• Aging. Changes associated with aging are not excluded from a role in the pathophysiology of meibomian gland dysfunction. Research has found acinar atrophy in human meibomian glands without gland distention, which implies a primary cause of secondary atrophy not related to meibum build-up.13,14 These changes may in fact happen due to aging as it occurs throughout other organs in the body, leading to decreased meibomian gland secretion and increased symptoms of dryness.
• Digital devices. With the ubiquity of computers, tablets and smart phones, the latest addition to the pathophysiology behind meibomian gland dysfunction is what was once called “computer vision syndrome.” The main culprit: decreased blink rate. On average, a person blinks around 15 times per minute. Studies show that during computer use, the blink rate decreases by 60% to about 4.5 blinks per minute.15 As discussed earlier, the action of blinking releases the meibum from the meibomian gland, delivering it to the lid margin. With less frequent blinking, meibum build-up creates similar long-term issues with acinar and meibomian gland atrophy.15,16
• Demodex folliculorum and Demodex brevis. Demodex colonization of the lid margin can contribute to MGD. D. folliculorum live in the lash follicles where they attach to the eyelashes, consume epithelial cells and contribute to lash misdirection and loss. They also cause microabrasions with their claws, triggering epithelial hyperplasia and hyperkeratinization, which accounts for the classic appearance of the collaratte around the eyelash base.
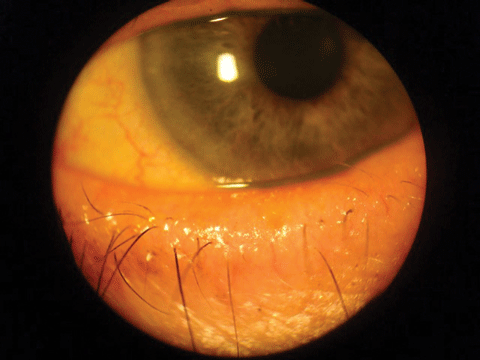 |
| Eyelid thickening and lash loss. Click image to enlarge. |
D. brevis live deep within meibomian glands, obstructing the orifice and creating an environment where meibum builds up, creating similar secondary effects as in that lead to meibomian gland dysfunction.
Demodex also act as a vector for bacteria such Staphylococci and Streptococci, which can trigger an additional inflammatory cascade.17
When assessing meibomian gland function, it is important to consider the elements that can contribute MGD, given its nature as progressive condition, and manage them accordingly. Any risk factors that increase evaporative stress and loss of meibomian gland function can lead to the sequelae of dry eye, including inflammation, pain and blurred vision along with the functional and anatomical changes.
| Meibography of advanced meibomian gland atrophy seen by the lack of the white, column-like glands nasally and the truncation of many temporally. |
Tests that evaluate MG function include the meibomian gland expression, lipid layer thickness (LLT) and, indirectly, tear film break up time (TBUT). During MG expression, the number of functional glands is measured on the lower lid margin, and the type of MG secretions—from liquid oil to granular secretions, to no secreting glands—is also measured. The lipid layer thickness can be measured with in-office interferometry (available on the Lipiview II) with thinner scores indicating an increased the risk of having meibomian gland dysfunction. Tear film break up time can easily be performed to assess for evaporative dry eye.
Conclusions
Meibomian gland dysfunction is one of the hottest topics in eye care today. Having a firm understanding of the role of meibomian gland structure and function is crucial to adequately addressing the all-too-common condition we call dry eye. If we look at the key words in the definition of meibomian gland dysfunction, the terms terminal duct obstruction and changes in glandular secretion are notable signs of dysfunction. By understanding the proper function of the meibomian gland, we can address, improve and promote a healthy ocular surface, ultimately improving the lives of our patients, who are often desperate for our help.
Is it time for us to rethink our dry eye diagnostic and treatment philosophy? The answer may very well be yes. By looking specifically at the function of the meibomian gland, we no longer have to rely on the patients’ subjective symptoms to determine both the time at which intervention can begin, and the course of management.
With increased attention to the meibomian glands, the root cause of DED can be identified and treated, leaving your patients with the quality of ocular health—and life—that they deserve.
Dr. Olennikov is a recent graduate of Pacific University’s College of Optometry and an optometric resident at Virginia Eye Consultants in Norfolk, Virginia. After residency, she will be joining Virginia Eye Consultants with a focus on vitreoretinal disease, peri-operative care and clinical research.
Dr. Cunningham is the Director of Optometry at Dell Laser Consultants in Austin, Tx.
Dr. Whitley is the Director of Optometric Services at Virginia Eye Consultants in Norfolk, Va.
|
1. Behrens A, Doyle JJ, Stern L, et al. The Dysfunctional Tear Syndrome Study Group. Dysfunctional Tear Syndrome: A Delphi Approach to Treatment Recommendations. Cornea. 2006 Sep;25(8):900-7. 2. Management and Therapy of Dry Eye Disease: Report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop. Geerling G, Pflugfelder S, Kinoshita S, et al. Ocular Surface. 2007 Apr;5(2)163-78. 3. Nichols KK, Foulks GN, Bron AJ et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922-9. 4. Knop E, Knop, N, Millar T, et al. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Invest Ophthalmology & Vis Sci. 2011. 52(4);1938–78. 5. Sullivan D. Androgen Influence On The Meibomian Gland. Investigative Ophthalmology & Visual Science. 2000;41(12):3732-42. 6. Macsai M. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (An AOS Thesis). Transactions of the American Ophthalmological Society 2008;106:336. 7. Norn M. Expressibility of meibomian secretion: relation to age, lipid precorneal film, scales, foam, hair and pigmentation. Acta Ophthalmol (Copenh). 1987;65(2):137–42. 8. Suhalim, J, Parfitt, G, Xie, Y, et. al. Evaporative stress affects meibomian gland function. The Ocular Surface. 2014;12:59-68. 9. Whitson J, Varner D, Netland P. Glaucoma drugs and the ocular surface. Review Of Ophthalmology. 2006. Web. 4 Apr. 2016. www.reviewofophthalmology.com/content/d/features/i/1299/c/25020/#sthash.QNTcIbRD.dpuf. 10. Meyer MC, Rastogi P, Beckett CS. et al. Phospholipase A2 inhibitors as potential anti-inflammatory agents. Current Pharmaceutical Design, 2005;11(10):1301-12. 11. Dougherty JM, McCulley JP. Bacterial lipases and chronic blepharitis. Invest Ophthalmol Vis Sci. 1986;27(4):486-91. 12. Arita R, Itoh K, Inoue K, et al. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009;116(3):379–84. 13. Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002; 21(Suppl. 2):S70–4. 14. Obata H, Horiuchi H, Miyata K, et al. Histopathological study of the meibomian glands in 72 autopsy cases (in Japanese). Nippon Ganka Gakkai Zasshi. 1994;98(8):765–71. 15. Abelson, Mark. Review Of Ophthalmology. It’s time to think about the blink. www.reviewofophthalmology.com/content/d/therapeutic_topics/c/28665. 16. Wu, Huping et al. Meibomian gland dysfunction determines the severity of the dry eye conditions in visual display terminal workers”. PLoS ONE 9.8 (2014): e105575. Web. 4 Apr. 2016. 17. Liu J, Sheha, H, Tseng SCG. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy and Clin Immunol. 2010;10(5);505-10. |

