Focus on RetinaJune's issue is our 13th annual retina report, which explores the latest in clinical research and innovation in the detection and management of retinal diseases. Check out the other featured articles here: |
With the ongoing growth in scientific knowledge about the genetic causes of ocular disease and the current developments in gene therapy, it is becoming increasingly important for optometrists to be up-to-date on the latest developments in genetic testing and genomics research.
Genetic testing can be beneficial for patients with an inherited retinal disease (IRD) to obtain a more accurate genetic diagnosis, provide a more precise prognosis and facilitate gene-specific treatment and management options. For instance, a confirmation of pathogenic RPE65 gene mutations is necessary for patients with retinitis pigmentosa (RP) or Leber’s congenital amaurosis to be eligible for Luxturna (Spark Therapeutics) gene therapy. There are dozens of ongoing interventional clinical trials, including gene therapy, gene editing and antisense oligonucleotide-based therapy, which require confirmation of a genetic diagnosis. Patients and family members can also benefit from genetic counseling and appropriate referrals for beneficial resources, such as support groups and low vision rehabilitation.
 |
| Whole blood, buccal swab and saliva sample collection tubes. Click image to enlarge. |
Genetic Testing
The process of complete genetic testing is much more involved than simply obtaining a saliva sample and shipping it to a lab. Unlike a COVID test, which provides a straightforward positive or negative result, there are more factors that play a role in inherited retinal diseases.
The American Academy of Ophthalmology’s (AAO) Task Force on Genetic Testing published Recommendations for Genetic Testing of Inherited Eye Diseases in 2014. The document recommends provider-ordered testing through a Clinical Laboratory Improvement Amendments (CLIA)–certified laboratory for conditions for which the causative gene(s) have been identified.1 Direct-to-consumer testing should be avoided.1 Furthermore, the document outlines five parts of genetic testing: “(1) the clinical determination that a genetic eye disease is likely to be present, (2) the molecular investigation of genomic DNA samples […] (3) the analysis of the resulting molecular data in the context of relevant published literature and public databases using appropriate statistical methods, (4) the interpretation of the data in the context of the clinical findings and (5) the counseling of the patient about the interpreted findings and their implications.”1
If considering implementing genetic testing within a clinical practice, optometrists should have a plan to address all five components.
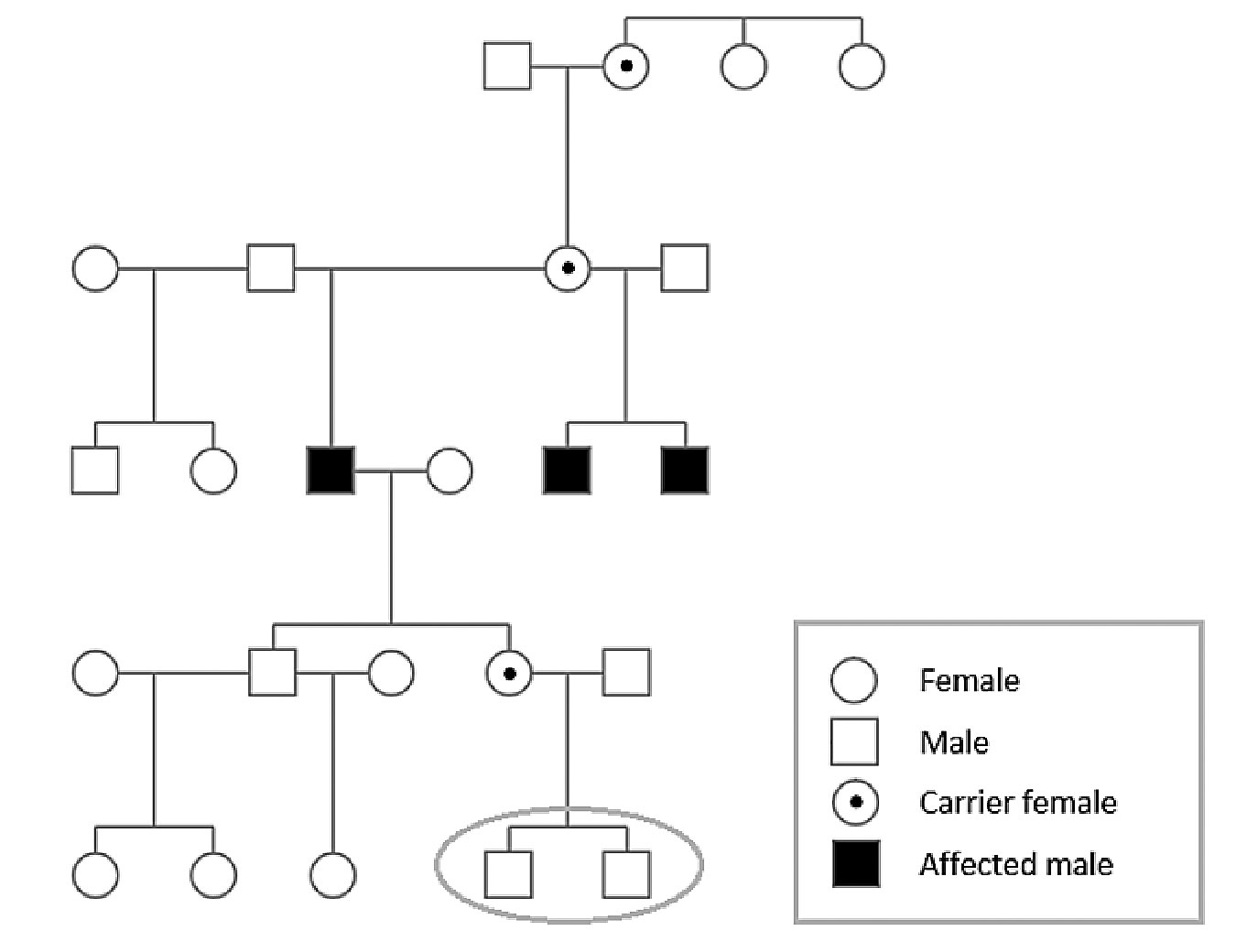 |
| Family pedigree of three brothers diagnosed with choroideremia confirmed with genetic testing. Applying the X-linked recessive inheritance pattern indicates that a daughter of one patient is a carrier for choroideremia; her two young sons each have a 50% probability of having inherited choroideremia. Click image to enlarge. |
Part 1: clinical exam. The initial examination is of course necessary to identify a tentative diagnosis or several differential diagnoses. Depending on the eye condition, the examination may include additional testing such as imaging, visual fields and electroretinography. The AAO’s Recommendations on Clinical Assessment of Patients with Inherited Retinal Degenerations provides a good outline of clinical testing for IRDs.2
It is important to use clinical examination and differential diagnoses to select an appropriate genetic test panel. The panel should include genes known to be associated with the differential diagnoses. For instance, a patient suspected to have X-linked RP should be tested with a panel that includes the many genes known to be associated with RP. In particular, the panel should be checked to include the RPGR gene, which accounts for a majority of X-linked RP cases.
However, testing more genes is not always better. In fact, it is recommended to order an inherited retinal dystrophy panel rather than whole-exome sequencing for a patient with clinical signs of an inherited retinal dystrophy. Ordering an appropriate panel increases the chances of a clinically useful result while limiting the possibility of unrelated secondary findings.
Parts 2 and 3: sample collection and laboratory analysis. There are many different laboratories and test panels to select from. One important recommendation is to work with a CLIA-certified laboratory. Two examples of commonly used test panels include the Blueprint Genetics Retinal Dystrophy Panel and the Invitae Inherited Retinal Disorders Panel. Both encompass 330+ genes associated with inherited retinal disorders. In addition to out-of-pocket and insurance billing options, these panels are currently supported by sponsored no-charge testing programs.
Specialized equipment for genetic testing in-office is minimal. Clinicians can request specimen collection kits directly from the testing laboratories. Saliva and buccal swab samples are the most commonly obtained in optometric practice. Saliva samples require the patient to spit in a tube and buccal samples typically require multiple cheek swabs. Patients should not eat, drink or chew gum prior to saliva sample collection.
Laboratory testing may take two to four weeks. The ordering practitioner and patient may be able to access the results through the respective laboratory’s online portal.
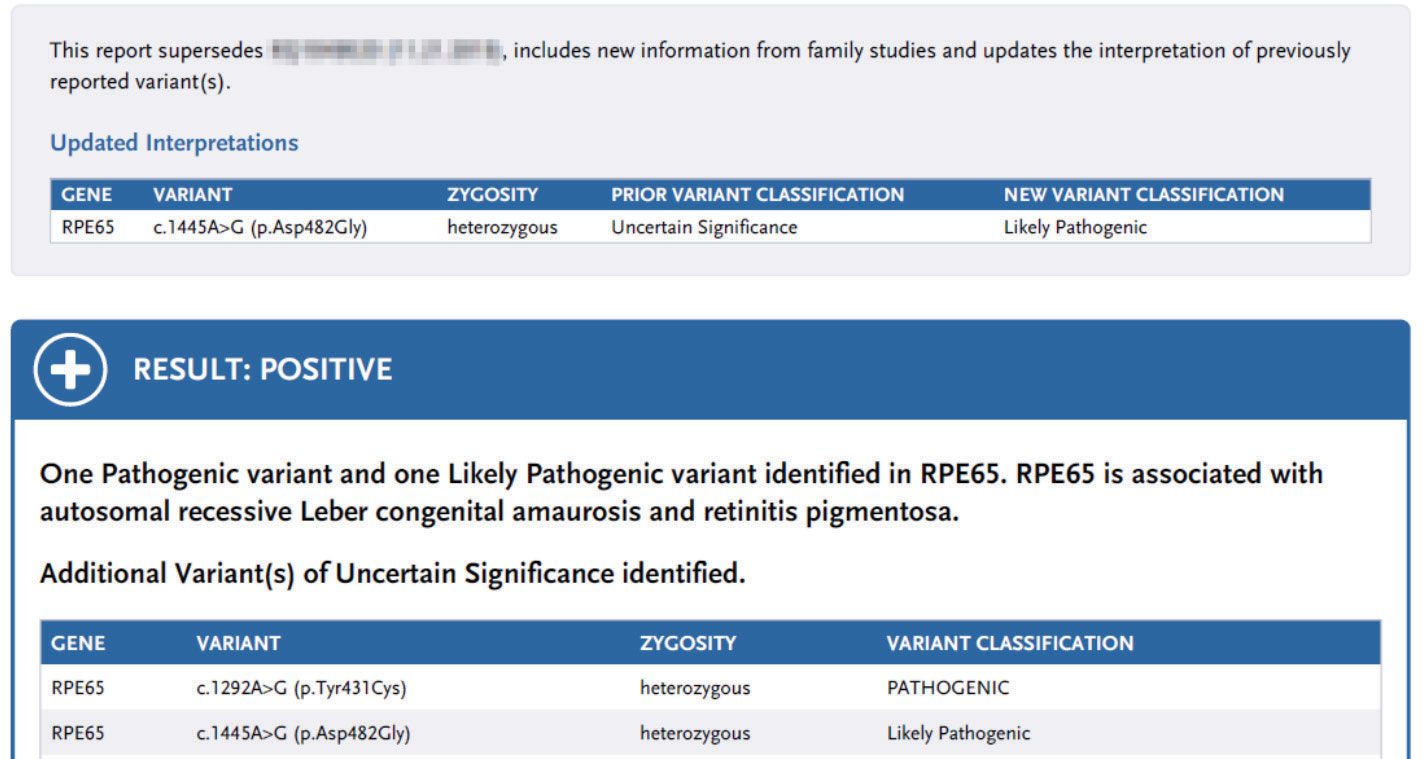 |
| Updated genetic test report for a patient identified with biallelic RPE65-associated RP. One variant was later classified as a variant of uncertain significance in the initial report; it was reclassified to likely pathogenic. This genetic test result helped qualify the patient for Luxturna gene therapy. Click image to enlarge. |
Part 4: genetic report interpretation in the context of clinical findings. Upon receiving the genetic test report, it is important to interpret the results within the context of the patient’s clinical findings, including ocular signs, medical history and family history.
A positive result indicates that a pathogenic variant or mutation was found in one of the genes tested. It is important to confirm if the identified pathogenic variant(s) correlate with the clinical diagnosis or if they are secondary findings unrelated to the condition in question. Keep in mind that a pathogenic variant must be present on both alleles of a gene to cause an autosomal recessive condition, whereas only one pathogenic variant must be present to cause an autosomal dominant condition.
A negative result merely indicates that no pathogenic mutations were found in the tested genes. Since genetic test panels are constantly growing and improving, retesting in the future may be indicated. Likewise, a patient with a negative result obtained several years ago with a limited test panel may benefit from updated testing.
Genetic test results may also indicate several variants of uncertain significance. These are periodically reclassified as we gain more knowledge about variants. Therefore, genetic test reports are not “static” and may be updated over time. The practitioner may need to counsel the patient as changes are made and reports are updated.
A family pedigree can be useful to confirm the inheritance pattern if needed. Likewise, if genetic testing confirms diagnosis of a condition with a known inheritance pattern, the pedigree may be useful for identifying at-risk relatives. Familial variant testing may be indicated and can be ordered after a variant of interest has been identified in the affected family member.
Part 5: patient counseling. It is important to allot sufficient chair time to discuss results and further management with the patient. This can include discussion of eligibility for FDA-approved gene therapy (currently only available for RPE65-associated retinal dystrophy), interventional clinical trials (gene therapy, gene editing, antisense oligonucleotide-based therapy, etc.) and natural history studies. The database clinicaltrials.gov is a great resource to search for current trials.
Inform patients about joining a research database such as the Foundation Fighting Blindness My Retina Tracker Registry to be updated on relevant research studies.
In addition to the patient education provided in-office, it is important to provide patients and family members access to counseling with a certified genetic counselor. No-cost genetic counseling may be available when using select sponsored panels.
Genetic Testing for AMDKeep in mind that genetic test panels for inherited retinal dystrophies do not test for the risk of developing AMD. There are genetic tests marketed specifically for AMD that aim to assess risk of developing the condition and to inform supplementation recommendations based on genotype. Publications have presented opposing views on the clinical utility of genetic testing for the purpose of determining supplementation.3,4 The American Academy of Ophthalmology currently does not recommend genetic testing for AMD.1 |
Counseling and Management
Genetic testing by itself may not adequately represent the entire clinical picture for any individual patient. Some conditions have large genotypic and phenotypic heterogeneity. As such, patients may have a similar phenotype or clinical presentation, but have different affected genes. For instance, there are over 60 different genes known to be associated with non-syndromic RP. On the other hand, some patients may have identical mutations on the same affected gene but have very different clinical presentations, even within the same family.
Furthermore, while the diagnosis and prognosis of some monogenic conditions can be easily predicted by one gene, other conditions are more complex and may be affected by a combination of multiple genes and/or environmental factors.
Therefore, prognosis and management should be individualized, taking into account both genetics and clinical findings. Once a patient’s specific pathogenic mutation(s) have been identified, literature searches of recent research can be used to provide the most up-to-date management recommendations. For instance, lifestyle modifications such as smoking cessation are recommended for certain conditions.
Patients identified with syndromic conditions may benefit greatly from increased screening, monitoring and medical management of the known systemic risks associated with their condition. For instance, a child diagnosed with Joubert syndrome would benefit from being monitored for kidney disease and a patient diagnosed with Stargardt’s disease may be advised to avoid vitamin A supplementation.
Confirmation of a progressive vs. nonprogressive condition and the projected impact on vision can be crucial for counseling the patient and family on prognosis and future management. This can help direct planning for appropriate low vision aids and assistive technology.
Regardless of the diagnosis, remind patients that annual eye exams are still important to monitor eye health. Even if there is no currently available FDA-approved treatment for the genetic cause of a patient’s particular eye condition, there may be a clinical trial in the near future. Regularly scheduled exams also allow patients to be updated on new and upcoming advances in treatments and low vision aids.
Patients with vision loss may greatly benefit from low vision services to maintain independence and be prescribed devices for school, work and activities of daily living. For example, a patient with RPGR X-linked RP would benefit from eye exams and referrals to monitor for cataracts, find out about the latest in assistive technology and receive information on the several ongoing RPGR clinical trials.
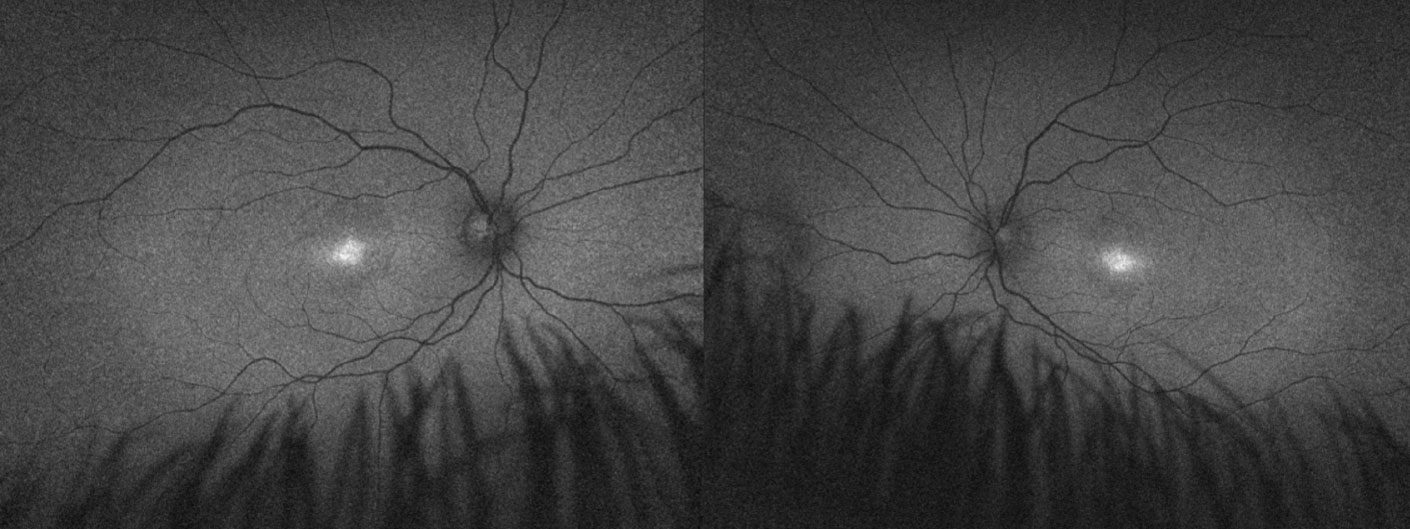 |
| Fundus autofluorescence imaging of a child with achromatopsia confirmed with genetic testing. The diagnosis helped provide the family with assurance of likely stable vision and appropriate recommendations for school, including prescribed low vision aids and discontinuation of Braille training. Genetic test results were also used to refer the patient for an achromatopsia gene therapy clinical trial. Click image to enlarge. |
Implementing Testing in Practice
Genetic testing for IRDs can be implemented in optometric practice with relatively little equipment. Regardless, management of rare dystrophies can still be daunting in a practice that does not regularly see this patient population. It is advised to keep in mind the extensive amount of counseling and chair time needed to provide comprehensive care for patients with IRDs.
Thankfully, there are a growing number of patient and doctor resources such as the Foundation Fighting Blindness Open Access Genetic Testing Program (www.fightingblindness.org/open-access-genetic-testing-program) and the Spark Therapeutics ID Your IRD Gene Testing Initiative (www.invitae.com/en/idyourird). Currently, these programs cover the cost of genetic testing lab fees and provide free genetic counseling for qualified patients with inherited retinal dystrophies. Foundation Fighting Blindness offers genetic counseling through InformedDNA, and ID Your IRD offers genetic counseling through Invitae. Without these programs, the cost of testing can be a significant barrier for patients.
If offering genetic testing through a sponsored program, optometrists must check that the patient meets the program’s eligibility criteria. Optometrists can bill for exam fees and any associated testing but cannot charge for the sponsored lab testing.
Optometrists should consider the scope of practice and any relevant laws governing lab testing and genetic testing within their state. Also, educate staff members on genetic testing policies as well as privacy and confidentiality concerns with regards to genetic information.
Clinical Pearls for In-Office Testing
|
Patient Preparation
Informed consent and patient education are important parts of genetic testing. It is important to ask patients their goals for genetic testing and to discuss reasonable expectations.
For example, if a patient diagnosed with cone dystrophy is interested in genetic testing because they heard about a gene therapy “cure for blindness,” it would be important to discuss that there is no current cure for cone dystrophy. However, genetic testing is indicated to help the patient learn more about the genetic cause of their cone dystrophy and may qualify them for future clinical trials.
Patients should be informed that genetic testing may not provide a definitive answer; the genetic test result is only one component of the clinical picture. Even with our current test panels, a causative mutation may be identified in 60% to 80% of patients with inherited retinal dystrophies.2 Depending on the eye condition, there can be a significant possibility that the test may not yield a positive result. For instance, approximately only three-quarters of cases of Leber’s congenital amaurosis can be attributed to causative mutations.2 Nonetheless, genetic testing for these patients is still highly recommended for the possibility of being positive for RPE65 gene mutations and qualifying for gene therapy. On the other hand, the known genes associated with achromatopsia account for almost all cases. Therefore, a patient with achromatopsia tested with a comprehensive panel is almost certain to receive a positive test result.
Patients with a family history of IRD but without any signs or symptoms may inquire about genetic testing. In this case, it is more effective to first test the affected family member to identify the causative mutation(s) before testing unaffected individuals who are interested in carrier status.
Testing is indicated when the possible benefits outweigh the potential cons. Inform patients about the possible implications of genetic test results for the patient and family members. Special considerations need to be taken into account when testing children and minors. For instance, clinicians and parents may need to weigh delaying testing to respect a minor patient’s autonomy against the potential benefits of earlier testing for particular adult onset conditions.
Patients should be made aware of the increased possibility of secondary findings when running broader genetic tests. Patients may also need to be informed about the implications of genetic testing on insurance, as genetic discrimination protections vary by state. Various publications, such as the American Medical Association’s Code of Medical Ethics, can serve as an ethics guidance resource for clinicians.
Validity and Utility of IRD Testing
There are three considerations to note when assessing the accuracy and value of genetic testing: analytical validity, clinical validity and clinical utility.
Analytical validity is how well a test can accurately detect the presence of the genetic variants. It is recommended to use CLIA-certified labs to ensure analytical validity. Additionally, confirming that the panel selected includes the genes known to be associated with the differential diagnoses will help ensure an appropriate genetic test.
Clinical validity refers to how accurately the genetic variant predicts presence, absence or risk of the ocular condition. Clinical validity can vary depending on the condition in question. Some genes are associated with multiple different inherited retinal conditions; therefore, diagnosis will rely heavily on other clinical testing. Some conditions exhibit variable expressivity or reduced penetrance, meaning some patients with the pathogenic mutation may present with a much milder presentation compared to other patients with the same mutation, or without any signs or symptoms at all.
Clinical utility refers to whether the test provides meaningful clinical impact to patient management. As we learn more about the genetic basis of inherited retinal dystrophies and as we develop more treatments, the clinical utility of genetic testing will only continue to grow.
Key Takeaways
Optometrists play an important role in educating patients with IRDs about the possibilities of genetic testing, ongoing clinical trials and the development of future treatments. Either conducting genetic testing in-office or referring patients for testing, optometrists can use genetic information to provide valuable insight for diagnosis and prognosis for patients with IRDs.
Genetic test results when combined with clinical examination findings and genetic counseling can help patients understand inheritance risks and inform important treatment decisions. Identifying a patient’s pathogenic variants is crucial for determining eligibility for gene therapy and genotype specific clinical trials.
Dr. Lin is a full-time assistant professor at the Southern California College of Optometry at Marshall B. Ketchum University. She conducts genetic testing and treats patients in the clinical departments of Low Vision Rehabilitation and Acquired Brain Injury at the University Eye Center at Ketchum Health in Anaheim, CA. She is a Fellow of the American Academy of Optometry. Dr. Lin is a consultant for Applied Genetics Technologies Corporation and Spark Therapeutics ID Your IRD Speakers’ Bureau.
1. Stone EM, Aldave AJ, Drack AV, et al. Recommendations for genetic testing of inherited eye diseases-2014. American Academy of Ophthalmology. www.aao.org/clinical-statement/recommendations-genetic-testing-of-inherited-eye-d. February 2014. Accessed April 15, 2022. 2. Duncan JL, Bernstein PS, Birch DG. Recommendations on clinical assessment with inherited retinal degenerations-2016. American Academy of Ophthalmology. www.aao.org/clinical-statement/recommendations-on-clinical-assessment-of-patients. June 2016. Accessed April 15, 2022. 3. Awh CC, Hawken S, Zanke BW. Treatment response to antioxidants and zinc based on CFH and ARMS2 genetic risk allele number in the Age-Related Eye Disease Study. Ophthalmology. 2015;122(1):162-169. 4. Chew EY, Klein ML, Clemons TE, et al. No clinically significant association between CFH and ARMS2 genotypes and response to nutritional supplements: AREDS report number 38. Ophthalmology. 2014;121(11):2173-80. |

