 |
History
A 77-year-old black female presented with a chief complaint of decreased vision in her left eye beginning four weeks prior, which she first noticed while reading. She said the vision loss was progressively worsening and that her glasses did not alleviate the symptoms at distance or near. She did not note any pain or discomfort.
Her systemic history was remarkable for hypertension and chronic obstructive pulmonary disease, for which she was appropriately medicated with systemic oral preparations and a steroid inhaler. Her ocular history was remarkable for open angle glaucoma, which was controlled with a topical prostaglandin drop. She had peripheral iridotomies in both eyes, nuclear sclerosis in her right eye and was pseudophakic in her left eye.
Diagnostic Data
Her best-corrected entering visual acuities were 20/25 OD and 20/60 OS at distance and near with no improvement upon pinhole. Her external examination was normal, and showed no evidence of an afferent pupillary defect. Biomicroscopic examination demonstrated patent peripheral iridotomies with no new anterior segment abnormalities. Goldmann applanation tonometry measured 12mm Hg OU. The new pertinent findings in her left eye are revealed in the optical coherence tomograph (OCT).
Your Diagnosis
Does this case require any additional tests? What does this patient’s history and clinical findings tell you about her likely diagnosis? How would you manage this patient?
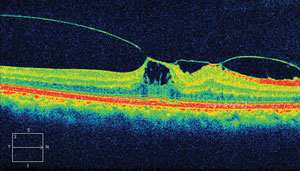 | |
| This OCT displays findings from our 77-year-old patient’s left eye. What diagnosis does the image suggest? |
Discussion
Additional studies included color fundus photography, indirect stereo-biomicroscopic 90D examination of the fundus along with Amsler grid testing and documentation.
The diagnosis in this issue is Vitreomacular traction syndrome. Posterior vitreous detachment (PVD) refers to the separation of the cortical vitreous from the internal limiting membrane (ILM) of the retina anywhere posterior to the vitreous base.1-14 It may be localized, partial, or complete.3,5 Symptoms include the episodic appearance of floating spots in the visual field, particularly noticeable against the bright blue sky or pastel colored surfaces. The floating spots are the result of shadows cast by incompletely liquefied vitreous tissue suspended and drifting through the liquefied portions. The result is the characteristic entoptic phenomenon known as “floaters”, “clouds” or “smoke”. The phenomenon is often accentuated in environments having fluorescent illumination.7 In cases where floaters are the result of acute vitreoretinal with secondary vitreous hemorrhage, the floating spots may be large. In the event the vitreous hemorrhage is on the visual axis a reduction of acuity may be present.13,14 In cases where there is incomplete detachment and retinal traction, the symptom of flashing lights (photosia) may occur.7-9 PVD has no racial or gender predilection and is common, occurring in approximately 50% of patients over the age of 50, increasing to approximately 75% by age 65.8,11,12 There is an increased risk for PVD in aphakic or pseudophakic eyes, myopes and eyes with a history of trauma or intraocular inflammation.9,12 Women may be prone to PVD at a younger age secondary to reduced hyaluronic acid synthesis.11 Reduced hyaluronic acid synthesis has been associated with decreasing postmenopausal estrogen levels.2,8,11
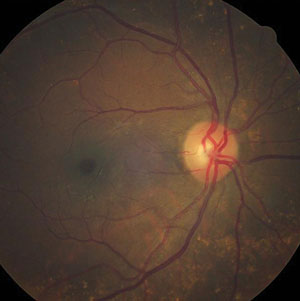 | |
| Can this fundus image help identify the pathology behind our patient’s reported vision loss? |
Biomicroscopic examination of the tissue reveals an optically clear space filled with liquefied vitreous between the detached posterior hyaloid and the retina.1,5,10 The pathognomic sign of a PVD is the presence of a clinically observable fibrous annulus of tissue (Weiss or Vogt ring) overlying the optic disc.9 This ring represents the remnants of the circular attachment of the posterior, primary cortical vitreous to the site encircling the nerve (Area of Martegiani).10 The presence of a Weiss ring does not indicate total separation of the posterior hyaloid membrane from the ILM, nor does its absence confirm that the posterior hyaloid membrane remains attached.4 Acute presentations may present with preretinal or vitreous hemorrhage (VH).13,14 While patients on anticoagulation therapy may have a larger incidence of VH following acute PVD their risk for retinal tears and detachments does not seem to be any higher than that of the general population (approximately 46%).13,14
In cases where the detachment is incomplete, traction is produced by tangential mechanical forces caused by focal condensations and shrinkage of the vitreous.15,16 The vitreous traction can be independent of ocular movements or static. Dynamic traction occurs when centripetal forces during eye movements increases the chances of retinal tears and rhegmatogenous detachments. When tractional forces are sustained they induce stress on the posterior retina causing vitreoretinal/macular traction syndrome.15-17 These patients may experience variable changes in visual acuity and metamorphopsia which has the potential to worsen as the tissue distorts.16,17
The vitreous is comprised of water, inorganic salts, ascorbic acid and two major macromolecules: collagen and glycosaminoglycans (GAGs-particularly hyaluronic acid).18-22 The vitreous “gel” is formed by a dilute meshwork of collagen fibrils that provides a scaffold-like structure that is “inflated” by hyaluronic acid.18 Attachments of the vitreous to the retina occur in areas where the ILM is the thinnest. Attachment locations include the vitreous base, the margins of the optic disc (when this area detaches it produces the classic circular Weiss or Vogt ring), the back of the crystalline lens in contact with the hyloidocapsular ligament of Wieger, the 500-µm diameter foveola, along large retinal vessels and sites of abnormal vitreoretinal adhesion such as lattice margins.1,5,7 It was previously thought that the posterior vitreous collagen fibrils directly inserted into the ILM but recent findings suggest that an extracellular matrix composed of laminin, fibronectin and sulfated proteoglycans interface and act as a “molecular glue”.6,8 The balance of the posterior vitreous adherence is more diffuse.8
As aging occurs progressive reorganization of the hyaluronic acid and collagen molecules induces two major vitreous changes, liquefaction and aggregation of collagen fibrils.11,22 Synchysis refers to liquefaction of the vitreous and is typically a senile process accelerated by myopia, inflammation, trauma, hereditary vitreoretinal syndromes such as Stickler and Marfan syndromes, retinal vascular diseases, aphakia, and vitreous hemorrhage.1,11,20-24 Synchysis is the most common degenerative change in the vitreous and has been found to be present as early as 4 years of age. Liquefied vitreous may account for approximately 20% of the vitreous volume by ages 14–18 years.1,3 The degeneration continues steadily after age 40 with more than half of the vitreous body becoming liquid by the age of 80 years.2,11,19 The process of synchesis leaves pockets of liquefaction known as lacunae. Biomicroscopically, they are regions that develop centrally, devoid of collagen fibrils. These lacunae typically enlarge and coalesce over time.3,11,22,24,26 Syneresis refers to the process of vitreous collapse where collagen fibrils aggregate into macroscopic bundles of parallel fibrils.1,11,19,22,27
When both synchysis and syneresis are present, the collagen aggregates become suspended and mobile within the lacunae.22 Obeying the same optics seen in models that predict the shadows during an eclipse of the sun, they create moving penumbra which, when large enough, can be detected by the patient. The aggregates can also be observed clinically as freely moving dark particles in the vitreous that scatter with ocular movement.1,5,22
The process of PVD begins with synchysis of the vitreous and weakening of the vitreo-retinal adhesions.19-24 Enlargement of formed lucunae cause the posterior vitreal cortical wall overlying the involved area to become thinned.20 As the vitreo-retinal adhesions dissolve, discontinuities form within the posterior hyaloid (either via fissure evolution or via a microbreak in the thin cortical vitreous layer).28 This allows synchytic vitreous to enter the subhyaloid space dissecting the posterior hyaloid from the ILM of the retina.1-5,10-22 PVD typically begins in a single superior perifoveal quadrant. The vitreoretinal ILM attachments at the fovea and optic nerve head often remain stubbornly attached.29 Over time, as the perifoveal detachment enlarges, it completely surrounds the attachments at the fovea.29 Finally, detachment of the vitreous from the foveal region produces a funnel shaped configuration with attachments at the optic disc and vitreous base.27-29 When the PVD releases from the optic nerve the process is complete.29-31 A complete PVD occurs when the posterior cortical vitreous is detached from the entire retina, including its attachment to the optic nerve up to the posterior border of the vitreous base.32 Research has demonstrated that even healthy young eyes may begin to form incomplete or partial PVD beginning as early as the fourth decade of life.31 These cases may remain asymptomatic but progress slowly for years before becoming a complete PVD.31 Additionally after PVD occurs, autopsy studies have shown that nearly half of all eyes with a PVD will have residual vitreous tissue on the inner retinal surface – a condition called vitreoschisis.33 This remnant vitreous may proliferate to form an epiretinal membrane which is composed of glial cells and histiocytes, forming a scaffolded connection between the retina and remaining vitreous cortex.33
An anomalous PVD (APVD) results when synchysis occurs without sufficient detachment from the ILM. Here, gel liquefaction exceeds the degree of vitro-retinal dehiscence (separation).19 Due to the elastic properties of the vitreous this results in static anterior tractional effects at the interface.19 Those with genetic collagen disease, such Marfan’s, Ehlers-Danlos and Stickler’s syndromes have a higher incidence of APVD.11,19,34 The physics of anomalous PVD has the potential to generate forces which split the posterior vitreous cortex causing vitreoschesis.19 When this phenomenon occurs in the periphery, tractional forces increase the risk of retinal tears and detachments.17,19 When it occurs in or adjacent to the macula, it has the potential to induce wrinkling of the neurosensory retina referred to as macular pucker. The pathology is also known as cellophane maculopathy, epiretinal membrane, vitreoretinal interface maculopathy and vitreo-macular traction syndrome (VMTS).17,19,35 Vitreoeschisis may contribute to the process of macular hole formation and increase the risk diabetic macular edema.17,35 Other associations appearing concurrently with VMT include age-related macular degeneration and retinal vein occlusion.36 When vitreoschesis occurs in the region of the optic disc, vitreo-papillary traction may increase the risk of neovascularization of the disc.19 In susceptible patients, the process can increase the risk of proliferative vitreoretinopathy.19 It is estimated that the prevalence of VMTS is low in the U.S. population. However, 1.5% of the population has other eye disease which is either caused by or associated with VMTS.37
Uchino and coworkers have proposed a grading system for age-related PVD: Stage 1, incomplete perifoveal PVD in up to three quadrants; Stage 2, incomplete perifoveal PVD in all quadrants, with residual attachment to the fovea and optic disc; Stage 3, incomplete PVD over the posterior pole with residual attachment to the optic disc and Stage 4, complete PVD.31
Recently, The International Vitreomacular Traction Study Group Classification of Vitreomacular Adhesion, Traction, and Macular Hole study has organized, based on OCT, diseases of the vitreomacular interface.33 VMT is defined by evidenced detachment of the perifoval vitreous cortex from the retinal surface with a macular attachment within a 3mm radius of fovea and association of attachment with distortion of the foveal surface, intraretinal structures and/or elevation of the fovea above the RPE without interruption of the inner retinal layers.33 VMT is further classified by weather it is found in isolation or with concurrent retinal conditions and by size.33 Focal VMTS is defined as being less than or equal to 1500 micrometers and broad VMTS is defined as being greater than 1500 micrometers.33
The management for PVD and acute PVD is thorough examination of the posterior segment to rule out the presence of retinal holes, tears and detachments. The vitreous should be evaluated for “tobacco dust”, sometimes referred to as Schaffer’s sign (retinal pigment epithelial cells or red blood cells that are ejected into the vitreous following a retinal tear) and evidence of vitreous hemorrhage.37-39 Dilated directed indirect biomicroscopy, three-mirror lens and binocular indirect ophthalmoscopy should all be considered to rule out concurrent retinal pathology requiring immediate treatment. Patients should be educated to the classic signs and symptoms of retinal detachment: repetitive flashing lights, sudden shower of additional new floating spots, cob webs in the field of vision and missing visual field as if a curtain was blocking the view. Since patients initially diagnosed as having uncomplicated PVD have approximately a 3.4% chance of a retinal tear within the first 6 weeks following the event they should be advised to limit their activity (no contact or competitive sports, no weight lifting, no jogging or running) over that period until they can be re-evaluated. Patients should be counseled that in most cases the symptoms disappear on their own. In the event symptoms remain and create a distracting annoyance, pars plana vitrectomy can be discussed as an option.40
B-scan ultrasonography is another useful tool for differentiating posterior vitreous detachment and its sequellae from other retinal pathologies.41 It has been used with a high rate of success to confirm suspected diagnosis, assisting in the record keeping and guiding potential retinal management.41
The advent of the OCT makes for easier identification and diagnosis of vitreoretinal disorders such as VMT. As such, over time the incidence of VMTS is expected to increase as the technology becomes more widespread. Patients with asymptomatic VMT should be observed for at least 2-3 months; nonoperative treatment with ocriplasmin can be considered when disorders persist; surgery is recommended if VMT-related symptoms and loss of function is significant.42 In a study by Theodossiadis et al. it was found that spontaneous VMT resolution is negatively associated with greater horizontal adhesion diameter as uncovered by OCT.43 The strength of the traction exerted by the vitreous on the fovea seems to be positively associated with the angle of the vitreomacular interface.43 Because spontaneous resolution is rare, worsening symptoms and/or persistence longer than 6 months should be evaluated for intervention.
The average time of complete posterior vitreous detachment occurs at an average of 15 months after initial presentation, but worsening signs via OCT and fundoscopy or progressive visual symptoms should warrant immediate considerations for treatment as earlier traction release is associated with the best chances for preserving visual potential.36,42-44 Eyes with thin anteroposterior strands attached to the center of the macula seem to have a favorable natural history.
Cystoid changes along with symptomatic visual loss may spontaneously resolve without any lasting signs or deformation of retinal tissue; however it is more common to observe some residual dysfunction and permanent distortion.15
Some final notes: 1. The Weiss ring which results from a PVD may be complete (circular) or broken and often casts a shadow during indirect ophthalmoscopic examination, 2. Patients with posterior vitreous detachment with vitreous pigment granules or hemorrhage are 52 times more likely to have a retinal tear compared with those who have normal findings on qualitative vitreous examination. These patients should be seen more frequently after the intial presentation (1 week, 3 weeks, 5 weeks as compared to 3-4 weeks in cases without this finding), 3.Patients should be educated to the potential long-term complications of pars plana vitrectomy for the removal of floaters (potential for cataractogenesis in phakic patients, potential for prolonged healing course, potential for macular edema).
Dr. Gurwood thanks Dr. David Domachowski for his contributions to this case.1. KhuKrana, AK. The Vitreous. In: KhuKrana, AK. Comprehensive Ophthalmology. Daryaganj, Delhi, India, New Age International 2007:243-248.
2. Sebag J. Development and aging of the vitreous. In: Sebag J. The vitreous: structure, function, and pathobiology. New York: Springer-Verlag; 1989:73-92.
3. Le Goff MM, Bishop PN. Adult vitreous structure and postnatal changes. Eye. 2008;22(10):1214–1222.
4. Snead MP, Snead DR, James S, Richards AJ. Clinicopathological changes at the vitreoretinal junction: posterior vitreous detachment. Eye (Lond). 2008;22(10):1257-62.
5. Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149(3):371-82.
6. Serpetopoulos CN, Korakitis RA. An optical explanation of the entoptic phenomenon of 'clouds' in posterior vitreous detachment. Ophthalmic Physiol Opt. 1998;18(5):446-51.
7. Hollands H, Johnson D, Brox AC, et al. Acute-onset floaters and flashes: is this patient at risk for retinal detachment? JAMA. 2009;302(20):2243-9.
8. Sharma S, Walker R, Brown GC, Cruess AF. The importance of qualitative vitreous examination in patients with acute posterior vitreous detachment. Arch Ophthalmol. 1999;117(3):343-6.
9. Margo CE, Harman LE. Posterior vitreous detachment. How to approach sudden-onset floaters and flashing lights. Postgrad Med. 2005;117(3):37-42.
10. Tiedeman J, Mittra R, Han D, et al. Vitreous and retinal detachments. In: Regillo C, Brown G, Flynn H. Vitreoretinal disease: the essentials. New York: Thieme; 1999: 65-86.
11. Majcher CE, Gurwood, A.S. The role of the vitreous in retinal disease. Review of Optometry Retinal Supplement 2012; 149(4): 6s-14s.
12. Smiddy WE, Michels RG, Glaser BM, deBustros S. Vitrectomy for macular traction caused by incomplete vitreous separation. Arch Ophthalmol. 1988;106(1)624–8.
13. El-Sanhouri AA, Foster RE, Petersen MR, et al. Retinal tears after posterior vitreous detachment and vitreous hemorrhage in patients on systemic anticoagulants. Eye (Lond). 2011;25(8):1016-9.
14. Witmer MT, Cohen SM. Oral anticoagulation and the risk of vitreous hemorrhage and retinal tears in eyes with acute posterior vitreous detachment. Retina. 2012 Oct 26. [Epub ahead of print] doi: 10.1097/IAE.0b013e3182671006.
15. Hikichi T, Yoshida A, Trempe CL. Course of vitreomacular traction syndrome. Am J Ophthalmol. 1995;119(1):55-61.
16. Kampik A. Pathology of epiretinal membrane, idiopathic macular hole, and vitreomacular traction syndrome. Retina. 2012;32 Suppl 2:S194-8.
17. Wylegała E, Woyna-Orlewicz A, Piłat J, et al. Traction maculopathies--pathogenesis and diagnostics. Klin Oczna. 2006;108(10-12):457-63.
18. Schubert H, Kincaid M, Green R, et al. Anatomy and physiology. In: Regillo C, Brown G, Flynn H. Vitreoretinal disease: the essentials. New York, Thieme, 1999: 3-25.
19. Sebag J. Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease.Graefes Arch Clin Exp Ophthalmol. 2004;242(8):690–8.
20. Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149(3):371–382.
21. Skeie JM, Mahaian VB. Dissection of the human vitreous body elements for proteomic analysis. J Vis Exp. 2011:23(47)2455-64.
22. Sebag J.Age-related changes in human vitreous structure. Graefes Arch Clin Exp Ophthalmol. 1987;225(2):89-93.
23. Maumenee IH. Vitreoretinal degeneration as a sign of generalized connective tissue diseases. Am J Ophthalmol 1979;88(9):432– 449.
24. Sebag J. Pathology of the vitreous. In: Sebag J. The vitreous: structure, function, and pathobiology. New York, Springer-Verlag,1989: 97–147.
25. Bishop PN. Structural macromolecules and supramolecular organization of the vitreous gel. Prog Retin Eye Res. 2000;19(3):323-44.
26. Bishop PN, Holmes DF, Kadler KE, et al. Age-related changes on the surface of vitreous collagen fibrils. Invest Ophthalmol Vis Sci 2004;45(4):1041–1046.
27. Bos KJ, Holmes DF, Kadler KE, et al. Axial structure of the heterotypic collagen fibrils of vitreous humour and cartilage. J Mol Biol 2001;306(5):1011–1022.
28. Smiddy WE, Michels RG, Greene WR. Morphology, pathology, and surgery for idiopathic macular disorders. Retina. 1990;10(1):288–96.
29. MacDonald HR, Johnson RN, Schatz H. Surgical results in the vitreomacular traction syndrome. Ophthalmology. 1994;101(1)1397–403.
30. Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vitreomacular traction syndrome. Br J Ophthalmol. 2002;86(8):902-9.
31. Uchino E, Uemura A, Ohba N. Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated byoptical coherence tomography. Arch Ophthalmol. 2001;119(10):1475-9.
32. Halfter W, Winzen U, Bishop PN, et al. Regulation of eye size by the retinal basement membrane and vitreous body. Invest Ophthalmol Vis Sci. 2006;47(8):3586-94.
33. Duker, JS., P. Kaiser, S. Binder, M.D. de Smet. et al. The International Vitreomacular Traction Study Group Classification of Vitreomacular Adhesion, Traction, and Macular Hole Ophthalmology. 2013. 120(12):2611–2.
34. Maumenee IH. Vitreoretinal degeneration as a sign of generalized connective tissue diseases. Am J Ophthalmol 1979;88:432– 449.
35. Bottós J, Elizalde J, Arevalo JF, et al. Vitreomacular traction syndrome. J Ophthalmic Vis Res. 2012;7(2):148-61.
36. David R.P. Almeida and Eric K. Chin. Spontaneous Resolution of Vitreomacular Traction in Two Patients with Diabetic Macular Edema Case Rep Ophthalmol. 2014;5(1):66–71.
37. Machemer R. Pathogenesis and classification of massive periretinal proliferation. Br J Ophthalmol. 1978;62(11):737-47.
38. Khan Utman SA, Mahomed I, Baig HM. A case of atypical idiopathic choroidal effusion syndrome. J Coll Physicians Surg Pak. 2012;22(6):406-8.
39. Gupta M, Prasad S. Acute posterior vitreous detachment. Br J Ophthalmol 2001;85(4):504.
40. Stoffelns BM, Vetter J, Keicher A, Mirshahi A. Pars plana vitrectomy for visually disturbing vitreous floaters in pseudophacic eyes. Klin Monbl Augenheilkd. 2011;228(4):293-7.
41. Zvornicanin J, Jusufovic V, Cabric E, et al. Significance of ultrasonography in evaluation of vitreo-retinal pathologies. Med Arh. 2012;66(5):318-20.
42. Shao L, Wei W. Vitreomacular Traction Syndrome. Chinese Medical Journal, Beijing Tongren Eye Center. 2014. 127(8):1566-1571.
43. Theodossiadis, GP, Grigoropoulos V, Theodoropoulou S, et al. Spontaneous Resolution of Vitreomacular Traction Demonstrated by Spectral-Domain Optical Coherence Tomography. American Journal of Ophthalmology. 2014;157(4):842-851.
44. Levy J, Klemperer I, Belfair N, et al. Rapid spontaneous resolution of vitreomacular traction syndrome documented by optical coherence tomography. International Ophthalmology. 2005;25 (1):247-25.

