Age-related macular degeneration (AMD) remains the leading cause of irreversible visual loss among the elderly in developed nations.1 In 2004, the prevalence of advanced AMD in the United States was estimated to be 1.47%, indicating approximately 1.75 million patients, and was projected to increase to 2.95 million by 2020.2 Depending on one’s definition of the disease, it will affect about one in three people older than 75 years of age.3
We are all familiar with the 2001 National Eye Institute’s (NEI) Age-Related Eye Disease Study (AREDS). This double-masked randomized clinical trial (RCT) evaluated the effects of nutritional supplementation on the progression of AMD, and found that treatment with antioxidants plus zinc was associated with a statistically significant reduction in disease progression in patients with intermediate or advanced AMD—by about 25% after five years.4 This was exciting news for anyone in the business of caring for patients with macular degeneration.
Before this trial, all we had to offer to patients were Amsler grids for home monitoring, consultations on smoking cessation, protecting the eyes from UV radiation and improvements in diet. Having a treatment that would reduce disease progression in intermediate or advanced disease by 25% transformed the way we practice and the recommendations we are obligated to make to best serve our patients and practice according to our oath. (“I will advise my patients fully and honestly of all which may serve to restore, maintain or enhance their vision and general health,” from The Optometric Oath.)
The Age-Related Eye Disease Study 2 (AREDS2), a subsequent RCT, was then performed to determine if adding lutein plus zeaxanthin, DHA plus EPA, or both to the AREDS formula would further reduce the risk of advanced AMD. From the results of this trial, the National Eye Institute recommended replacing the beta carotene in the original formula (over concerns of increasing the risk of lung cancer in smokers) with 10mg of lutein and 2mg of zeaxanthin.22 This substitution to the original AREDS formula resulted in an additional beneficial effect of about 20% beyond the effects of AREDS in reducing the progression to advanced AMD.
Even with AREDS2 therapy, patients vary substantially in disease progression rates, which suggests a potential pharmacogenetic component in treatment response.
Pharmacogenetics is the study of inherited genetic differences in drug metabolic pathways, which can affect an individual’s response to drugs, both in terms of therapeutic effect as well as adverse results.
The Genetics of AMD
AMD is not a Mendelian (or monogenetic) disorder like Stargardt disease or X-linked retinitis pigmentosa, in which the development of clinically detectable disease is very likely if a disease-causing genotype is present in a single gene. Rather, AMD is considered a complex genetic disease where the presence of one or more risk alleles does not necessarily result in a more affected phenotype, and the absence of risk alleles does not necessarily result in a less affected phenotype.23,24
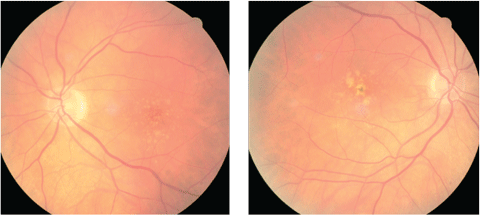 |
| At left, this eye qualifies as intermediate AMD per the AREDS classification system. This is a patient who would benefit from supplementation per the AREDS data. In the image at right, the patient qualifies as intermediate AMD and is progressing to the advanced stage due to the pigment clumping centrally. Click images to enlarge. |
In AMD, the genotypes of many genes appear to interact with each other and with the environment to determine whether a patient will develop clinically significant disease.24 However, of all the human multi-genetic diseases, AMD has been shown to have the strongest genetic contribution.25
The Human Genome Project catalogued and identified single nucleotide polymorphisms (SNPs), which are genetic variations that influence disease risk. Since then, SNPs involving at least 19 genes have been shown to be involved in AMD.
Of those 19 genes, two major susceptibility genes for AMD—CFH and ARMS2—have been shown to have a substantial contribution (perhaps 80%) to the heritability of AMD.26 Complement factor H (CFH) is the main regulator of the alternate complement pathway of the immune system, and multiple independent genetic studies have shown that dysfunction of the complement system is a key factor in AMD development.27 The age-related maculopathy susceptibility 2 (ARMS2) gene is involved with energy metabolism in the mitochondria.26
Despite advances in genetic research, the American Academy of Ophthalmology’s Task Force on Genetic Testing currently recommends against routine testing for AMD until specific treatment or surveillance strategies are shown in prospective studies to benefit those with specific genotypes.24,28
Pharmacogenetics and AREDS
Recent pharmacogenetic studies using the original AREDS data have reported differences in treatment outcomes with respect to variants in genes for CFH and ARMS2.
In 2008, Michael Klein, MD, co-director of the Macular Degeneration Center at the Casey Eye Institute, and colleagues published a study using AREDS data that showed an observed interaction between CFH and antioxidants plus zinc.29 This analysis was based on genetic data from 876 white participants of the original AREDS trial who had intermediate AMD or unilateral advanced AMD at baseline. Using multivariate analysis, and after controlling for age, sex, education, smoking and body mass index (BMI), researchers reported a greater reduction effect in AMD progression in patients with the CFH non-risk genotype compared to the CFH risk genotype. However, antioxidants plus zinc reduced progression to advanced AMD (compared to placebo) in all six genotype subgroups tested. Neither zinc alone or antioxidants alone were superior to antioxidants plus zinc in any genotype subgroup; therefore, the researchers did not recommend genetic screening.29
How Much Zinc is Enough (or Too Much)?The concentration of zinc in the eye is higher than in most tissues of the body. Zinc is a cofactor for enzymes involved in visual function, and it plays an important role in regulating enzymes that are involved in the oxidative process. One major problem in assessing the need for zinc in AMD is the lack of information about its biological role in the retina and surrounding structures.5 Like other tissues, the retina can be damaged by too much or too little zinc.6,7 At least one study has found that levels of zinc are reduced in human eyes with signs of AMD.8 Depletion of zinc increases oxidative stress, may cause deficits in phagocytic and lysosomal functions, and may induce macro-molecule synthesis and caspase-dependent apoptosis—mechanisms that are all implicated in AMD.5,7,9,10,11 Zinc depletion also markedly increases the vulnerability of retinal pigment epithelial cells to UV radiation through UV-induced DNA damage.12 In addition, inflammation has been associated with AMD and we know that zinc supple- mentation raises plasma zinc concentration, which then boosts the immune system and provides better protection in AMD and in general aging.5,13,14,15 So, the role of zinc depletion and its consequences have been established—and now recent research indicates that excess zinc may be just as harmful in AMD.5 Researchers have shown that drusen are filled with anomalous deposits of zinc, some of which are free or weakly protein bound.5,16 This discovery led to the hypothesis that while zinc (along with antioxidants) has been shown to be protective during the intermediate to late stage of AMD (as was shown in the AREDS trial), excessive levels may contribute to the development of AMD at the early stages.5,16,18 A 2013 paper clarified a potential molecular mechanism for zinc-induced drusen formation.18 This study show excess zinc induces precipitation of immune factors that may contribute to the development of drusen and reduce the progression to advanced AMD in higher risk patients.18 Obviously, more research needs to be done to further elucidate the role of zinc regulation or dysregulation in retinal tissue and AMD. It could be possible that the pathway to drusen formation and from drusen to advanced disease could be quite different.18 In the AREDS2 trial, the version of AREDS2 with reduced zinc was shown to be as efficacious as the AREDS2 formula with higher zinc, and is therefore arguably the safer choice in a well-nourished population. The recommended dietary allowance (RDA) for zinc in adults is 12mg to 15mg/day. However, some elderly people appear to require zinc intakes above the RDA in order to maintain positive zinc balance.19 Elderly people below the poverty line or people with certain food preferences (vegetarians or people who only eat fish or chicken) are likely to be zinc deficient.5 Therefore, the AREDS2 formulation with the original zinc dosage would be a better choice in this population. Of note, copper is necessary with long-term high zinc supplementation above the RDA to avoid a copper/zinc imbalance. Supplementation with quantities of zinc above the sug- gested upper limit can result in copper deficiency, suppress the immune system, increase the risk for metastatic prostate cancer and impair behavior.5,20 Interestingly, the original AREDS formulation provides copper in the form of cupric oxide. However, animal studies have shown that the bioavailability of this form of copper “is not significantly different from zero.”21 Practitioners who are still recommending the original AREDS formula or AREDS2 with the higher zinc levels (80mg vs. 25mg) to all patients with AMD without taking into consid- eration the stage of the disease, or the nutritional status of the patient, could potentially be doing more harm than good. My recommendation as an optometrist as well as a certified nutrition consultant: Consider the nutritional status of the patient and opt for an AREDS2 formulation with lower zinc and a form of copper other than cupric oxide when indicated. |
In 2013, Carl C. Awh, MD, and colleagues at Tennessee Retina analyzed AREDS data but reached different conclusions.30 These authors studied 989 white AREDS patients with intermediate AMD in at least one eye. They estimated AMD progression rates for nine CFH/ARMS2 genotypes. Using multivariate analysis, and after controlling for age, sex, education, smoking and BMI, the researchers reported that patients had statistically significant differences in outcomes based on CFH and ARMS2 genotypes. For 23% of patients, the original AREDS formulation was the best treatment. For 49% of patients, a formulation other than AREDS was more beneficial. For 13% of patients, the AREDS combination was harmful and accelerated vision loss significantly faster than placebo, which was thought to be due to an excess of zinc.30
Following this publication, the AREDS investigators (NEI’s Emily Y. Chew, MD, and colleagues) reported in 2014 an “unplanned retrospective evaluation” of 1,237 white AREDS study participants (AREDS Report Number 38).31 The AREDS investigators tested the response to the original AREDS supplement using genotypes described by Klein et al. and then by Awh et al. They dissented from the study results from Awh et al. because they found no significant interactions, and concluded that AREDS supplementation reduces the rate of progression across all genotype groups.31
Awh et al. responded by disputing AREDS Report Number 38, claiming that the 27 comparisons made by Chew et al., many with small sample sizes, were not clinically interpretable.31,32
Awh et al. subsequently analyzed 989 white participants from the same AREDS trial and defined four categories of risk variants based on alleles at CFH and ARMS2. Their findings showed that patients with a high CFH risk and no ARMS2 risk treated with the AREDS formula had a 135% increased AMD progression compared to those treated with placebo. Also, patients with a low CFH risk allele and a high ARMS2 risk allele had a 37% decreased AMD progression if treated with the AREDS formula compared to placebo.
They presented their research at the American Society of Retinal Specialists annual meeting in September 2014 and then later published their findings in 2015.32 They recommended using genotype-directed nutritional supplementation for high-risk patients, specifically to identify the highest risk patients—those homozygous for CFH without ARMS2 risk alleles.
Dueling Statisticians
Most recently, Chew et al. have responded, criticizing Awh et al. for presenting results that they characterize as biased, and therefore difficult to interpret.33 Awh et al. had acknowledged the following in their conclusions: “Validation by an independent data set would be helpful, but no such data set exists, and a replication trial would take years.”30,32 However, a data set was recently made available to the NEI AREDS researchers. Chew et al. had access to an additional 526 patients from the AREDS trial with the same qualifications used by Awh et al., a residual cohort whose DNA had recently become available. The demographics from the Awh et al. test data and Chew et al. validation sets are almost the same. If Awh et al. were correct in their conclusions, the findings from the residual cohort would serve to validate them.
Chew et al. analyzed the 526 patients in the residual cohort using the same four genetic groupings developed and used by Awh et al., and their findings did not, in fact, validate the findings by Awh et al. They reported that the combination of antioxidants plus zinc was beneficial in all genetic subtypes described by Awh et al.33
Based on the latest findings by Chew et al., the NEI concluded that because the findings by Awh et al. were not verified by the results of the residual cohort, genetic testing prior to treatment with AREDS supplements is not recommended.33
This debate between Awh et al. and Chew et al., which has played out in meetings and journals for several years, has caused much confusion and frustration for clinicians. Beyond the disagreements over data, claims of potential conflict of interest have been made on both sides.34 Such allegations—whether warranted or unwarranted—further muddle the arguments being made by introducing another dimension to the debate.
Clinical Applications
Both sides of the debate are quick to point out flaws in the other side’s data analysis hinging on such esoteric issues as “Bonferroni corrections for multiple comparisons.”
So, how should clinicians—who are not experts in trial design, genetics, or statistical analysis—interpret these conflicting results? Information from three credible resources may provide assistance in answering this question.
The first source, a 2015 review from Bascom Palmer Eye Institute, concluded that the balance of available evidence does not support the use of genetic screening to guide clinical decisions in AMD patients at this time.35 The Bascom Palmer doctors pointed out that many of the subgroups analyzed on both sides of the debate were too small for statistical significance. They also noted that retrospective subgroup analysis is not the same thing as a prospective RCT, such as the AREDS trial. The likelihood of inadvertent selection bias, resulting in statistically significant but clinically meaningless associations, increases with the number of subgroups. They further pointed out that it was the original AREDS formula and not AREDS2 that was used in the studies.
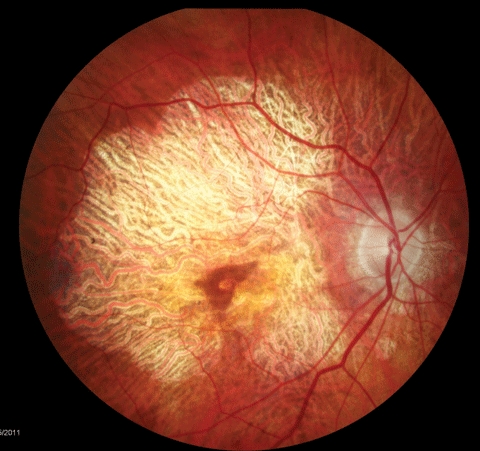 |
| Dry AMD patients require ongoing education about nutrition and other influences on their ocular status. Click image to enlarge. |
The second source is an article by Edwin Stone, MD, PhD, a researcher and professor at the University of Iowa Carver College of Medicine with over 25 years’ experience in studying the interplay between genes and eye disease. Dr. Stone is the director of the University’s Center for Macular Degeneration and the Nonprofit Genetic Testing Laboratory. He also headed the AAO’s task force on genetic testing.
In a May 2015 article in JAMA Ophthalmology (“Genetic Testing for Age-related Macular Degeneration Not Indicated Now”), he wrote, “I think that it is very important for all ophthalmologists (and optometrists) to recognize that the burden of proof of this hypothesis (that specific genotypes are associated with different responses to antioxidants plus zinc) lies with Awh and his colleagues; there is no burden of disproof for the AREDS investigators or, for that matter, anyone else in the scientific community. I continue to recommend AREDS vitamin supplementation to my patients with AMD, regardless of their genotype. I believe that all hypotheses about the clinical utility of genetic testing for AMD should be tested in a prospective fashion, with participants randomly assigned to groups that receive either conventional care or genotype-guided care. If, in such a prospective study, the clinical outcomes of the genotype-guided groups are significantly better than the clinical outcomes of the conventionally managed groups, this, and only this, will be meaningful evidence in favor of using genetic testing to help care for patients with AMD.”24
Dr. Stone reported no financial conflicts of interest. He does have a personal interest in AMD because both of his maternal grandparents lost substantial vision to the disease.
Recently, a report by University of Toronto biostatistician Rafal Kustra, PhD, in a Canadian online journal, concluded that the raw data in the NEI’s AREDS Report Number 38 actually supported the contention that 19% of patients had the genotypes that appear to fare worse on the supplement than placebo.34 In a personal communication, Dr. Stone noted that this study did not prompt a rethinking of his conclusions in the JAMA Ophthalmology article.36
The third source to illuminate the genetic testing and AMD supplementation question is a lecture given at the American Academy of Optometry’s annual meeting in October 2015 by Stuart Richer, OD, PhD, which presented slides from both Dr. Awh and Dr. Chew that contradicted each other’s research.37
Dr. Richer then summarized options for doctors. One point concerned the dose of zinc in the original AREDS formula and the negative associations for lack of efficacy for the homozygous CFH/null ARMS2 subgroup, noted by Dr. Awh and several other researchers and biostatisticians. Dr. Richer explained that, from the AREDS2 conclusions, lowering the zinc dosage did not statistically diminish the efficacy of the formula, albeit in an older and sicker group of patients compared to AREDS. Therefore, using a lower zinc formula is one option for doctors to consider. Another option, he noted, was to offer a one-time genetic test to monocular patients “to [possibly] avoid a zinc hyperimmune response in one-seventh of all high-risk AMD patients.”
All three sources pointed out the importance of pharmacogenetics and that more research needs to be done to further elucidate the very important role it will likely have in AMD.
As a clinician on the front lines caring for patients with AMD, the following considerations come to mind when deciding treatment:
• The original AREDS study was a prospective RCT that showed a benefit for patients with intermediate to advanced AMD.
• The AREDS2 formula with lutein and zeaxanthin was shown to have an additional beneficial effect beyond that of the original AREDS.
• The studies by Awh et al. and Chew et al. were conducted on the original AREDS formula with higher zinc levels.
• Lowering the zinc dosage in the AREDS2 formula did not decrease efficacy for the “average” patient.
Last but not least, consider your patient’s eye health in relation to their total well-being. Although some practitioners use genetic testing to guide follow-ups with their AMD patients, this should not be offered without counseling and consideration of the psychological risks. For example, fear or unfamiliarity of genetic testing could cause some patients with very low-risk genotypes to defer appointments, which could increase their risk of vision loss. Meanwhile, other patients with high-risk genotypes may worry for 20 years yet never go on to develop clinically significant vision loss. n
Dr. Poteet holds an MS in human nutrition, is a certified nutrition consultant and a fellow of the Ocular Nutrition Society. She currently practices in Atlanta, Ga.
|
1. Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Org. 2004; 82(11):844-51. 2. Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122(4):564-572. 3. Bressler NM, Bressler SB, West SK, Fine SL, Taylor HR. The grading and prevalence of macular degeneration in Chesapeake Bay watermen. Arch Ophthalmol. 1989; 107(6):847-852. 4. Age-Related Eye Disease Study Research Group. A Randomized, placebo-controlled trial of high-dose supplementation with vitamins C and E, beta-carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119(10):1417-1436. 5. Lengyel I, Peto T. Cure or cause: opposing roles for zinc in age-related macular degeneration. Exp Rev Ophthalmol. 2008; 3(1):1-4. 6. Donati G. Emerging therapies for neovascular age-related macular degeneration: state of the art. Ophthalmologi. 2007;221(6):366-77. 7. Hyun HJ, Sohn JH, Ha DW, et al. Depletion of intracellular zinc and copper with TPEN results in apoptosis of cultured human retinal pigment epithelial cells. Invest Ophthalmol. Vis. Sci. 2001; 42(2): 460-465. 8. Newsome DA, Miceli MV, Tate DJ, et al. Zinc content of human retinal pigment epithelium decreases with age and macular degeneration, but superoxide dismutase activity increases. J Trace Elements Exp Med. 1996; 8(4):193-199 (1996). 9. Miceli MV, Tate DJ Jr, Alcock NW, Newsome DA. Zinc deficiency and oxidative stress in the retina of pigmented rats. Invest Ophthalmol. Vis. Sci. 1999; 40(6):1238-1244. 10. Kennedy C, Rakoczy P, Robertson A, et al. Kinetic studies on phagocytosis and lysosomal digestion of rod outer segments by human retinal pigment epithelial cells in vitro. Exp Cell Res. 1994;210(2):209-14. 11. Schraermeyer U, Peters S, Thumann G, et al. Melanin granules of retinal pigment epithelium are connected with the lysosomal degradation pathway. Exp Eye Res. 1999; 68(2):237-245. 12. Ames BN. Micronutrient deficiencies. A major cause of DNA damage. Ann NY Acad Sci. 1999; 889, 87-106. 13. Hageman GS, Luthert PJ, Victor Chong NH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001; 20(6), 705-732. 14. Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc. 2000; 59, 541-552. 15. Clemons TE, Kurinji N, Sperduto RD. Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS Report No. 13. Arch Ophthalmol. 2004; 122(5), 716-726. 16. Lengyel I, Flinn JM, Petro T, et al. High concentration of zinc in sub-retinal pigment epithelial deposits. Exp Eye Res. 2007; 84(4), 772-780. 17. Suh SW, Jenson KB, Jenson MS et al. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res. 2000; 852(2), 274-278. 18. Ruodan N, Tetchner S, Rodriguez E, et al. Zinc-induced self-association of complement C3b and Factor H: implications for inflammation and age-related macular degeneration. J Biol Chem. 2013;288(26):19197-19210. 19. Burke DM, DeMicco FJ, Taper LJ, Ritchey SL. Copper and zinc utilization in elderly adults. J Gerontol. 1981; 36:558-563. 20. Maret W, Sandstead HH, Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006; 20(1), 3-18. 21. Baker DH. Cupric oxide should not be used as a copper supplement for either animals or humans. J Nutr. 1999; 129:2278-2279. 22. Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013; 309(19):2005-2015. 23. Hampton BM, Kovach JL, Schwartz SG. Pharmacogenetics and nutritional supplementation in age-related macular degeneration. Clin Ophthalmol. 2015; (9):873-876. 24. Stone EM. Genetic testing for age-related macular degeneration: not indicated now. JAMA Ophthalmol. Epub 2015 Marc 19. 25. Ma Q, Lu AY. Pharmacogenetics, pharmacogenomics, and individualized medicine. Pharmacol Rev. 2011 Jun; 63(2):437-459. 26. Ratnapriya R, Chew EY. Age-related macular degeneration—clinical review and genetics update. Clin Genet. 2013; 84:160-166. 27. Charbel IP, Chong NV, Scholl HP. The significance of the complement system for the pathogenesis of age-related macular degeneration-current evidence and translation into clinical application. Graefes Arch Clin Ophthalmol. 2011;249(2):163-174. 28. Stone EM, Aldave AJ, Drack AV, et al. Recommendations for genetic testing of inherited eye diseases: report of the American Academy of Ophthalmology task force on genetic testing. Ophthal 2012; 119:2408-10. 29. Klein ML, Francis PJ, Rosner B, et al. CFH and LOC387715/ARMS2 genotypes and treatment with antioxidants and zinc for age-related macular degeneration. Ophthalmology. 2008;115(6):1019-25. 30. Awh CC, Lane AM, Hawken S, et al. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmol. 2013;120(11):2317-2323. 31. Chew EY, Klein ML, Clemons TE, et al. No clinically significant association between CFH and ARMS2 genotypes and response to nutritional supplements: AREDS Report Number 38. Ophthalmology. 2014;121(11):2173-80. 32. Awh CC, Hawken S, Zanke BW. Treatment response to antioxidants and zinc based on CFH and ARMS2 genetic risk allele number in the Age-Related Eye Disease Study. Ophthalmology. 2015;122:162-9. 33. Chew EY, Klein ML, Clemons TE. Genetic testing in persons with age-related macular degeneration and the use of AREDS supplements: to test or not to test? Ophthalmology. 2015;122:212-4. 34. Blackwell T. U.S. agency earning millions from anti-blindness pill defends it after Canadians’ safety alert. Health. Epub 2015 Dec 14. 35. Hampton BL, Kovach JL, Schwartz SG. Pharmacogenetics and nutritional supplementation in age-related macular degeneration. Clin Ophthalmol. 2015;9:873-6. 36. Stone, E. Personal communication, March 17, 2016. 37. Richer SP. Genetic Testing and AMD. Lecture given at American Academy of Optometry Annual Meeting Oct. 2015. Retrieved from www.aao.convergence-us.com. |

