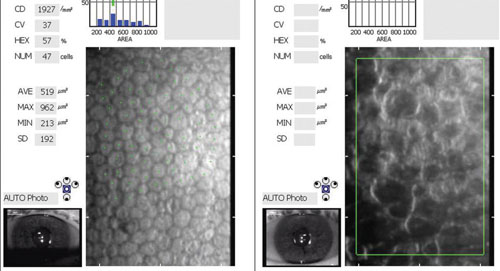
 |
| Images of corneal endothelium in an untreated eye with Fuchs' and one treated with DMEK. At left, the healthy donor endothelial cells form a regular hexagonal pattern. At right, it is difficult to discern viable endothelial cells. |
Regenerative medicine has been an increasingly successful method to treat disorders of the heart, pancreas and cartilage, but regeneration of the corneal endothelium has yet to reach a comparable stage. The commonly held dogma has long been that dystrophy is a death sentence for endothelial cells, which are notoriously non-duplicating and dormant.
This grim reality has prompted much innovation in surgical interventions to replace damaged endothelium with healthy tissue. In recent years, selective keratoplasties have emerged as a revolutionary approach to the treatment of corneal dystrophies, such as Fuchs’. But only recently have investigators begun to show that regeneration may indeed be possible. Seminal studies in Japan have indicated that corneal endothelial cells may have some proliferative capacity when appropriately stimulated. These researchers have explored the use of rho-kinase (ROCK) inhibitor drops as an alternative to corneal transplantation, which could greatly impact the way we treat and manage corneal diseases.
Corneal Dystrophies
The corneal classification system names five dystrophies of Descemet’s membrane (DM) and the endothelium—Fuchs’ endothelial corneal dystrophy (FECD), posterior polymorphous dystrophy (PPD), congenital hereditary endothelial dystrophy 1 (CHED1), congenital hereditary endothelial dystrophy 2 (CHED2) and X-linked endothelial corneal dystrophy (XECD).3
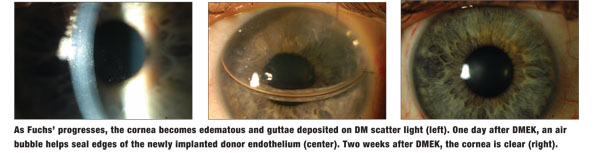
• Fuchs’, the most common corneal endothelial dystrophy, affects up to 2% of the US population. The distribution of the disease varies around the world, and it is typically rare in Japanese individuals. It is a progressive, late-onset, bilateral disease with autosomal dominant inheritance. Females are affected at 2.5 times the rate of males, for reasons that have not yet been elucidated.
Clinically, corneal guttae are the initial manifestation of FECD. These drop-like excrescences project from the posterior surface of DM. In the early stages, the cornea can also have a “beaten metal” appearance with pigment dusting on the endothelium. The guttae initially appear centrally and eventually coalesce. The endothelial cells die, reducing the endothelial cell density. Over time, both the pump and barrier functions become compromised, causing the cornea to swell and lose transparency. As the swelling progresses, epithelial microcysts can form and coalesce to become bullae.
Patients with Fuchs’ dystrophy suffer from reduced vision, which is typically worse in the morning due to increased stromal edema from overnight hypoxia. Because the guttae scatter light, FECD patients experience glare and photophobia, which can interfere with everyday tasks such as driving. This impairment of daily living activities under bright lighting is often more severe than would be expected based on measurements of visual acuity in a darkened exam lane. In the later stages, patients may experience tearing and pain from ruptured epithelial bullae.1-4
• Posterior polymorphous corneal dystrophy is also a dominantly inherited bilateral disease, but can have an asymmetric presentation. It presents in the second or third decade of life, with most patients being asymptomatic and stable. The abnormalities in PPMD occur at the level of DM and can take on different shapes: vesicle-like lesions, band lesions with a “railroad track” appearance and diffuse opacities.
The presence of vesicular lesions on DM is the hallmark of this dystrophy. Corneal edema is infrequent, but can occur and may be rapidly progressive. Another characteristic of PPMD is peripheral anterior synechiae; these can range from fine adhesions noted only with gonioscopy to large, broad-based membranes. The iris can be uninvolved or may exhibit broad areas of atrophy. PPMD shares similar clinical features with iridocorneal endothelial syndrome (ICE), so careful differentiation is needed. ICE syndrome is often unilateral, progressive and non-familial.
• Congenital hereditary endothelial dystrophy 1 & 2 are bilateral disorders that involve the entire endothelium at birth; the cornea is congenitally cloudy. They are rare, except in Saudi Arabia and southern India.
CHED1 is autosomal dominant, while CHED2 is autosomal recessive, more common and severe. Corneal clouding can range from diffuse haze to a ground-glass, milky appearance. Patients experience blurred vision, often accompanied by nystagmus.
• X-linked endothelial corneal dystrophy is more common in males. It presents as congenital corneal clouding with a milky ground-glass appearance. The gene mutation has not been identified.
Current Treatment Options
While patients can use hypertonic saline drops to reduce corneal edema during the early stages of FECD, transplantation is the definitive treatment for corneal endothelial dysfunction. In the 20th century, full-thickness penetrating keratoplasty (PK) was the only surgical option, whereas in the 21st century, targeted endothelial replacement through a technique known as endothelial keratoplasty (EK) has supplanted PK procedures. EK is safer and provides faster visual recovery.
Endothelial keratoplasty is performed through a small incision, similar to cataract surgery. The most frequently used technique is Descemet’s stripping endothelial keratoplasty (DSEK), which removes the central portion of DM and dysfunctional endothelium and replaces it with healthy DM, endothelium and posterior stroma taken from a donor cornea.5 The donor tissue is folded or curled for insertion through a small incision, then unfolded and pushed snugly against the back of the patient’s cornea with the use of an air bubble in the anterior chamber. This bubble dissipates over four to five days, leaving the DSEK attached. These grafts do not require any sutures, resulting in rapid visual recovery. Most patients can achieve a best-corrected vision of 20/25 to 20/40.
To further improve visual outcomes, progressive corneal surgeons have switched to a newer technique called Descemet’s membrane endothelial keratoplasty (DMEK), which replaces a patient’s diseased DM and endothelium with healthy DM and endothelium from a donor cornea.5,6 But in contrast to DSEK, DMEK does not include any donor stromal tissue.
Because a DMEK graft is extremely thin, it can be safely inserted through an even smaller incision than that used for DSEK (<2.9mm); smaller incisions are always prized in corneal surgery, as they induce less postoperative astigmatism. Using thinner donor tissue has improved visual outcomes as anticipated—sparing the host cornea from the addition of donor stroma reduces the potential for higher-order aberrations to affect visual acuity. Most DMEK patients achieve BCVA of 20/25 or better.
An unexpected benefit of DMEK is a reduction in the incidence of immunologic graft rejection episodes to less than 1% of cases. Because of the rapid and predictable outcomes and visual improvement with DMEK, surgeons are now able to treat the two eyes of a patient just one to two weeks apart—similar to modern cataract surgery.7 Corneal transplants have high success rates in patients with corneal dystrophy; five-year survival rates are 90% to 95%.8,9
Prospects for Topical Therapy
Is there anything on the horizon for treating diseases like FECD or corneal edema after injuries and cataract surgery without needing a transplant? A signaling molecule that may promote corneal endothelial cell proliferation, migration and adhesion is being investigated as a possible medical therapy for corneal dystrophy. Corneal endothelial cells are generally quiescent and non-replicating in adults; however, recent studies suggest that they do have some proliferative capacity when appropriately stimulated.
The signaling molecule under investigation is one of a family of molecules called ROCK inhibitors. These agents are protein serine/threonine kinases that influence cell migration, apoptosis (programmed cell death) and proliferation. In particular, ROCK signaling is thought to promote cell-cycle progression in various cell types, including corneal endothelial cells, although the underlying mechanism is unknown.
A Japanese team has been investigating the use of this molecule to promote proliferation of cultured corneal endothelial cells. In 2009, Naoki Okumura, MD, and associates found that inhibition of the ROCK pathway with the selective inhibitor known as Y-27632 could promote primate corneal endothelial cell survival in vitro.10 Primate corneal endothelial cells were harvested and cultured in a medium containing Y-27632 at a 10-µM concentration. It facilitated proliferation as well as adhesion of monkey corneal endothelial cells, and it inhibited apoptosis.
Subsequently, this same team demonstrated that following trans-corneal freezing to kill the central endothelium, injection of cultured endothelial cells along with application of an eye drop containing Y-27632 could regenerate the central endothelium and restore corneal clarity in monkeys. They next demonstrated that trans-corneal freezing followed by application of the ROCK inhibitor eye drops also could restore corneal clarity in monkeys, even without injection of cultured endothelial cells.11
In 2013, the same team reported results of the first study in eight human patients—four had central corneal edema and were diagnosed with Fuchs’ dystrophy, while the other four had diffuse pseudophakic corneal edema.11 Trans-corneal freezing of a 6mm area to kill the central corneal endothelium was followed by application of Y-27632 eye drops six times daily and gatifloxacin eye drops four times daily for seven days. Corneal clarity was restored in the four patients with central corneal edema, but not in the four with diffuse corneal edema.11
A subsequent paper reported longer follow up of one of the patients treated for Fuchs’ dystrophy.12 The vision in that 52-year-old patient improved to 20/16 and, 18 months after treatment, the endothelial cell density was 1,549 cells/mm2 centrally and 705 cells/mm2 peripherally. In early 2014, the Japanese team performed their first injections of cultured endothelial cells into three human eyes, followed by application of Y-27632 eye drops.13
These preliminary results have generated much excitement. Clearly, further safety studies are needed because cell proliferation, adhesion and migration normally are tightly regulated throughout the body to help prevent tumors and cancer development. Additional studies are also needed to determine the appropriate dosing strength and duration, as well as the optimal time to intervene in the disease course, especially with Fuchs’ dystrophy. The guttae that develop with Fuchs’ dystrophy are smaller and less prominent in Japanese eyes than they are in white eyes. Because the guttae scatter light, causing glare and photophobia, a surgical procedure to remove the guttae would be required in white eyes if the condition had already advanced to the stage of causing corneal edema or glare.
The possibility of a topical treatment for corneal endothelial dysfunction is compelling. Although ROCK inhibitors show promise, further safety studies and larger comparative studies are needed. Endothelial keratoplasty is currently the treatment of choice, providing patients with rapid visual recovery, with DMEK providing the best visual outcomes.
Dr. Kelley specializes in cornea and external disease, serving as the principal investigator for dry eye clinical trials.
Dr. Marianne Price is executive director of the Cornea Research Foundation of America in Indianapolis.
Dr. Francis Price Jr. is the founder and president of Price Vision Group and the Cornea Research Foundation of America.
1. Krachmer J, Mannis M, Holland E (eds.). Cornea. 3rd ed. Philadelphia, PA: Elsevier; 2011.
2. Bourne WM, Biology of the corneal endothelium in health and disease. Eye (Lond). 2003 Nov;17(8):912-8.
3. Weiss JS, Moller HU, Lisch W, et al. The IC3D Classification of the Corneal Dystrophies. Cornea. 2008 Dec;27 Suppl 2:S1-83.
4. Eghrari AO, Gottsch JD. Fuchs’ corneal dystrophy. Expert Rev Ophthalmol. 2010 Apr;5(2):147-159.
5. Price MO, Giebel AW, Fairchild KM, Price FW. Descemet’s Membrane Endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology, 2009 Dec;116:2361-8.
6. Guerra FP, Anshu A, Price MO, Price FW. Fellow eyes comparison of Descemet stripping endothelial keratoplasty and Descemet Membrane Endothelial Keratoplasty. Cornea 2011 Dec;30(12):1382-6.
7. McKee Y, Price MO, Gunderson L, Price FW. Rapid sequential endothelial keratoplasty with and without combined cataract extraction. J Cataract Refract Surg 2013 Sep;39(9):1372-6.
8. Sugar A, Tanner JP. Recipient risk factors for graft failure in the Cornea Donor Study. Ophthalmology 2009 Jun;116(6):1023-8.
9. Price MO, Fairchild KM, Price DA, Price FW. Descemet’s stripping endothelial keratoplasty: five-year graft survival and endothelial cell loss. Ophthalmology 2011 Apr;118(4):725-9
10. Okumura N, Ueno M, Koizumi N, et al. Enhancement of primate corneal endothelial cell survival in vitro by a ROCK inhibitor, Inv Ophthalmol Vis Sci 2009;50:3680-7.
11. Nakamura N, Koizumi N, Kay EP, et al. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2013 Apr 3;54(4):2493-502.
12. Koizumi N, Okumura N, Rho-associated kinase inhibitor eye drop treatment as a possible medical treatment for Fuchs’ Corneal Dystrophy. Cornea 2013;32:1167-9.
13. Personal communication with S. Kinoshita. March 1, 2014.

