History
A 52-year-old black male presented with an acute headache and diplopia that had persisted for two days. He described the headache as a sharp pain that was located around his right temple. While ibuprofen seemed to help dull the acute head pain, his headache returned when the medication wore off.
His ocular history was noncontributory, and he reported no history of trauma. His systemic history, however, was significant for a recent diagnosis of type II diabetes mellitus. The patient reported compliance with the prescribed medications. He reported no allergies of any kind.
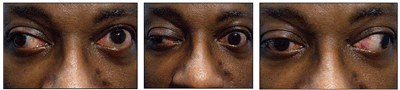
This patient with a history of type 2 diabetes presented with acute headache and diplopia.
Diagnostic Data
His best-corrected distance visual acuity was 20/25 O.D. and 20/20 O.S. with -0.50 sphere/+2.50D bifocals. External examination showed no afferent pupillary defect. Biomicroscopy uncovered normal and healthy anterior segment structures as well as open angles in both eyes.
His intraocular pressure measured 16mm Hg O.U. Dilated funduscopic examination revealed quiet posterior poles and distinct optic nerves, without evidence of disc edema. His cup-to-disc ratio measured 0.20 x 0.20 O.U.
Your Diagnosis
How would you approach this case? Does this patient require any additional tests? What is your diagnosis? How would you manage this patient? What’s the likely prognosis?
Thanks to Candice Tolud, Krutti Shah, M.D., Ahn T. Nugyen-Liang, O.D., and Mashel Nehema for their contributions to this case.
Discussion
When patients present with diplopia, always ask the following questions:
• Does the double vision go away when one eye is covered?
• Is the double vision up-and-down or side-by-side?
• Is the diplopia greater at distance or near?
• Is the event variable?
• Has this happened before?
• Is there associated head pain or pain upon eye movement?
In this case, we recorded our patient's extraocular muscle motilities in primary gaze and the four cardinal positions of gaze (up, down, left, right). Versions and ductions were compared and found to be equivalent. The patient's history made the forced duction test unnecessary.
We made a stat neuro-ophthalmologic referral to rule out infection, inflammation, space-occupying lesion or aneurysm. We also ordered a complete blood count with differential and platelets; lipid panel; angiotensin converting enzyme (ACE); fluorescent treponemal antibody absorption test (FTA-abs); reactive plasma reagin (RPR); Westergren erythrocyte sedimentation rate (ESR); and C-reactive protein. Additionally, we ordered magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) to rule out aneurysm and mass.
The diagnosis in this case is ischemic vascular cranial nerve palsy (CN III P).
Paresis of CN III can result secondary to any insult along its course––from the midbrain to the orbit.1-4 At the level of the midbrain in the brainstem, damage to the oculomotor fascicles causes incomplete paralysis due to the anatomic separation between the fasciles.4 Fasicular CN III P is usually secondary to ischemic conditions or metastatic lesions.3,4 Because of the proximity of adjacent structures, the following presentations are common:
• Benedikt’s syndrome. Benedikt's syndrome involves a lesion of the red nucleous and substantia nigra.4 Its symptoms include ipsilateral CN III P, contralateral hemichorea (uncoordinated movements), contralateral hemiballismus (loss of balance) and contralateral loss of sensation.4
• Nothnagel’s syndrome. Nothnagel’s syndrome affects CN III fascicles as they exit the cerebellar peduncle. It causes ipsilateral CN III P, dysmetria (poor spatial judgment) and contralateral ataxia (poor ability to judge the ground when walking).
• Claude’s syndrome. Claude's syndrome is a combination of Benedikt’s and Nothnagel’s syndromes. Claude's syndrome is caused by a dorsal midbrain lesion that results in ipsilateral third nerve palsy and contralateral ataxia.5
• Webber’s syndrome. Webber's syndrome involves the ipsilateral CN III fascicles and the descending pyramidal tract. It causes ipsilateral CN III P and contralateral hemiparesis.4
In adults more than 50 years of age who have hypertension or diabetes, pupil-sparing CN III P is assumed to be secondary to an ischemic vascular (vasculopathic) etiology, until proven otherwise.6,7
The clinical sign of “pupil sparing” is considered the hallmark finding in ischemic vascular CN III palsy.6,7 Ischemic vascular lesions are a result of microvascular disease, where the vascular architecture of the vasa nervorum is damaged. The resulting loss of function causes the eye to assume a "down-and-out" posture and creates a ptotic lid.6 The superficial axons that subserve pupillary constriction are fed by another blood supply, so they are unaffected, which preserves the pupilomotor response to light.6 Such findings are predisposed in patients with poorly controlled diabetes, hypertension, anemia and/or increased cholesterol levels.7
In isolation, this nonsurgical third cranial nerve palsy tends to resolve uneventfully within three to six months.6 If the palsy fails to resolve in this time, however, the MRI—along with other appropriate imaging—should be repeated to rule out additional etiologies.6,7
In the subarachnoid space, CN III is vulnerable to compressive insult from trauma or uncal herniation.8 The pupil is usually involved in these cases. Acute subarachnoid bleeding typically causes severe headache and reduced consciousness.8
The posterior communicating artery located at the junction of the carotid artery within the circle of Willis is a prevalent location of aneurysms that induce CN III P.9 Typically, aneurysms and subarachnoid hemorrhages cause pupil-involving CN III P secondary to external compressive and expansive effects that impinge on CN III axons.10 However, cases have been reported that demonstrate incomplete CN III palsies with pupil sparing. When the data is inconclusive or controversial, additional testing and investigation is warranted.11
In the intracavernous space, CN III travels in close proximity to CN IV and VI, the ophthalmic and maxillary divisions of CN V, and sympathetic fibers. Lesions in this region usually result in multiple nerve involvement. Intracavernous sinus lesions may result from cavernous sinus fistula, pituitary apoplexy, aneurysm, tumors, inflammation or infection.
Lesions in the orbit are usually accompanied by signs of inflammation, exophthalmos and/or mechanical restriction. Etiologies include trauma, neoplasm and infection. These lesions must be differentiated from muscle-affecting diseases, such as thyroid-related ophthalmopathy, ocular myasthenia gravis and ocular myopathy.12
The treatment of CN III P depends on the underlying cause. Tumors require resection or irradiation. Strokes require ischemic vascular therapy. Aneurysms currently have two treatment options––surgical clipping or endovascular coiling (EVC).13,14 Surgical clipping is the method of choice for closing the base of the aneurysm. When it is not surgically possible to reach the lesion, however, EVC is required. EVC is accomplished by passing a platinum catheter coil through the vascular system, which usually enters the system through a vessel in the leg. The coiling technique within the enlarged anomaly initiates blood clotting through a thrombotic reaction that stabilizes the malformation and prevents it from rupturing. 13,14 The risks associated with surgical clipping and endovascular coiling, such as stroke or death, are the same. Mortality rates are as low as 1% to 5%.11,12
As said earlier, vasculopathic CN III P often recovers spontaneously within three months. If it does not resolve within that time, further work-up, including MRI, vasculitis testing and CSF examination, is required. An MRI and MRA are immediately indicated to rule out the presence of emerging aneurysm in any case of CN III P that involves the pupil.13,14
As for our patient, the diagnosis of exclusion was made when the balance of the neurologic work-up was found to be negative. So, we referred the patient back to his primary care team for adjustment of his systemic treatment and followed him until resolution over a six-month period in both our office and the neuro-ophthalmology department at Wills Eye Hospital in Philadelphia.
1. Snell RS, Lemp MA. Cranial Nerves, Part I: Those Nerves Directly Associated with the Eye and Orbit. In: Snell RS, Lemp MA. Clinical Anatomy of the Eye, 2nd ed. Philadelphia: Blackwell, 1998; 294-328.
2. Moore KL, Dalley AF, Agur AM. Clinically Oriented Anatomy, 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2010; 902-7.
3. Shechtman DL, Woods AD, Tyler JA. Pupil sparing incomplete third nerve palsy secondary to a cavernous sinus meningioma: challenges in management. Clin Exp Optom. 2007 Mar;90(2):132-8.
4. Herzau V. Infranuclear Disorders of ocular motility. In: Schiefer U, Wilhelm H, Hart W. Clinical Neuro-Ophthalmology: A Practical Guide. New York: Springer, 2007; 137-54.
5. Fong CS. Claude's syndrome associated with supranuclear horizontal gaze palsy caused by dorsomedial midbrain infarction. Acta Neurol Taiwan. 2005 Mar;14(3):147-50.
6. Yanovitch T, Buckley E. Diagnosis and management of third nerve palsy. Curr Opin Ophthalmol. 2007 Sep;18(5):373-8.
7. Jacobson DM, McCanna TD, Layde PM. Risk factors for ischemic ocular motor nerve palsies. Arch Ophthalmol. 1994 Jul;112(7):961-6.
8. Kim SC, Chung J, Lim YC, et al. Oculomotor nerve palsy associated with rupture of middle cerebral artery aneurysm. J Korean Neurosurg Soc. 2009 Apr;45(4):240-2.
9. Santillan A, Zink WE, Knopman J, et al. Early endovascular management of oculomotor nerve palsy associated with posterior communicating artery aneurysms. Interv Neuroradiol. 2010 Jan;16(1):17-21.
10. Kim SC, Chung J, Lim YC, et al. Oculomotor nerve palsy associated with rupture of middle cerebral artery aneurysm. J Korean Neurosurg Soc. 2009 Apr;45(4):240-2.
11. Saito R, Sugawara T, Mikawa S, et al. Pupil-sparing oculomotor nerve paresis as an early symptom of unruptured internal carotid-posterior communicating artery aneurysms: three case reports. Neurol Med Chir (Tokyo). 2008 Jul;48(7):304-6.
12. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007 Jul;27(3):257-68.
13. King JT Jr, Berlin JA, Flamm ES. Morbidity and mortality from elective surgery for asymptomatic, unruptured, intracranial aneurysms: a meta-analysis. J Neurosurg. 1994 Dec;81(6):837-42.
14. Yanoff M, Duker J. Ophthalmology, 2nd ed. Philadelphia: Mosby, 2004;1324-34.

