 |
History
A 27-year-old Caucasian female reported to the office emergently with a chief complaint of painful vision loss in her left eye for a duration of two days. She explained that her eye became red and painful with “foggy” blurring of her vision.
Her systemic history was remarkable for migraine headaches—for which she had just started the medication Topamax (topiramate, 25mg) PO BID.
Her ocular history was unremarkable and she denied exposure to chemicals or allergies of any kind.
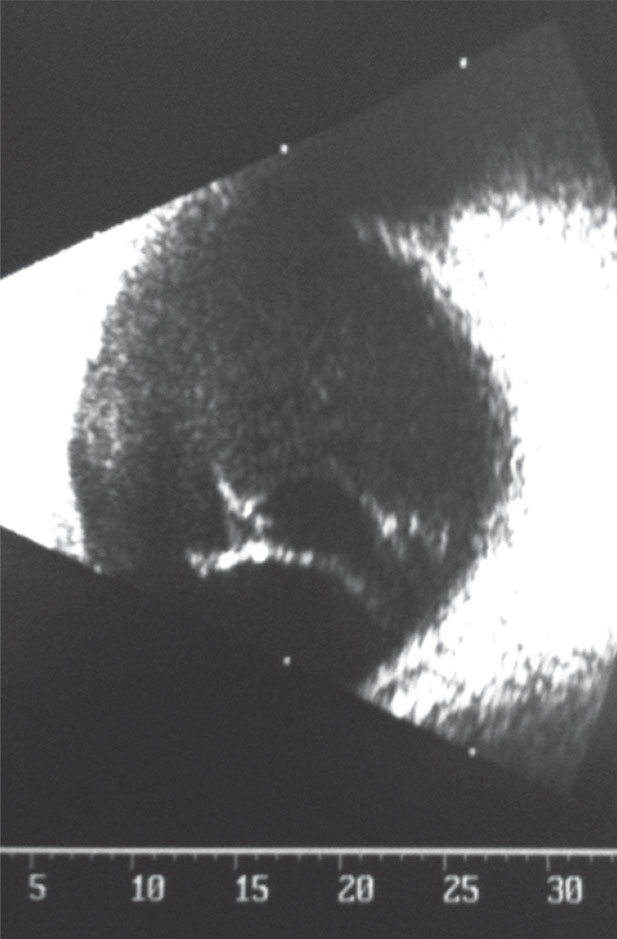 |
| This B-scan shows the right eye of our 27-year-old patient who had “foggy,” blurring vision. |
Diagnostic Data
Her best corrected entering visual acuities were 20/20 OD and 20/200 OS at distance and near. Her external examination demonstrated grade III conjunctival injection, tearing with mild corneal clouding in her right eye. No evidence of afferent pupil defect was seen. The biomicroscopic examination of the anterior segment of the right eye demonstrated conjunctival injection, corneal bullae, a narrow angle with shallow anterior chamber and evidence of cell and flare grade II.
The left eye was normal. Goldmann applanation tonometry measured 55mm Hg in the right eye and 15mm Hg in the left eye.
The B-scan of the patient’s right eye is available for review.
Discussion
Additional studies included 4-mirror gonioscopy with compression, sodium fluorescein staining to assess the magnitutde of the bullous keratopathy and non-dilated examination of the optic nerves. The medication topiramate was referenced for its published ocular complications leading to the B-scan to rule out ciliochoroidal effusion.
The diagnosis in this issue is secondary angle closure glaucoma via ciliochoroidal effusion syndrome precipitated by the use of topiramate. Topiramate is a sulfamate-substituted monosaccharide that is used to treat a variety of illness in both children and adults that includes epilepsy, bipolar disorder antipsychiotic medication obesity, general obesity and migraine headache.1-5
Generically, angle closure occurs when the peripheral iris physically contacts/covers the trabecular meshwork and corneal endothelium interrupting aqueous humor egress. When the mechanism is related to compacted anatomy (traditionally a short eyeball) resulting in iridolenticular touch (pupil block) aqueous passage from the posterior chamber to the anterior chamber becomes arrested inducing iris Bombay leading to the angle closure sequence previously described. This event is known as primary angle closure with pupil block.6 Secondary angle closures can occur when the angle is closed via pathologies that create the appositional sequence without blocking the pupil (angle closure without pupil block) and include posterior synechiae, iris neovascularization (rubeosis irides), aqueous misdirection syndrome (malignant glaucoma), plateau iris syndrome and ciliochoroidal effusion syndrome in the vicinity of the ciliary body.7-11
Ultrasound biomicroscopy has illuminated the mechanical mechanism for choroidal expansion in shallow choroidal effusions of the ciliary body as they create angle closure free from pupil block.11-15 The mechanism is anterior rotation of the ciliary body as well as forward movement of the iris and lens with subsequent shallowing of the anterior chamber and closure of the angle.11-15 As the choroid expands, choroidal and ciliary body edema (with possible choroidal effusion-transudate infiltration) places slack into the zonules; lens zonules with increased laxity produces increased convexity of the lens. Along with the forward and upward pushing of the ciliary body and iris, which creates the angle closure glaucoma, the net effect of increased lens convexity is refractive error shift with several diopters of acquired myopia. The clinical picture of choroidal expansion-induced angle closure differs from that seen in primary pupil block angle closure in that there is a flat anterior chamber without iris bombé.15 Choroidal expansion induced angle closure glaucoma as a result of administration of sulfa-based medications such as sulfonamide, acetazolamide, topiramate and hydrochlorothiazide is well documented.15-18 Topiramate (Topomax), which is used to manage chronic headache as well as induce weight loss has been implicated in choroidal expansion induced bilateral angle closure glaucoma along with induced myopia.14,17,18
One theorized mechanism proposed by early investigators was inflammatory sulfa-allergic reaction.15,17 Unfortunately, this mechanism is not accepted or supported as efforts to re-provoke the problem in the same patient failed when they were attempted.15,18 Investigators postulate that the ciliochoroidal effusion caused by sulphonamides results secondary to an idiosyncratic response in the uveal tissue.19 In essence, it is an unpredictable event triggered by immune mediated responses that is neither anaphylactic or dose dependent.19 Here, reactive drug metabolites bind to proteins, forming altered compounds, which are recognized by the host system as foreign substances, inciting immune reactions.19 A patient must receive a sensitizing dose prior to the inciting immune reaction which may occur with any subsequent dose. The risk of any adverse reaction to any sulfonamide is approximately 3%.19
Besides topiramate, other sulfonamides have been reported to participate in the clinical syndrome, including acetazolamide, sulfasalazine, hydrochlorothiazide, and indapamide.19 The literature, as a rule, recognizes all ocular sequellae caused by pharmacologic provocation as reversible if recognized early with minimal pharmacologic intervention and drug cesation.15-19
Treatment for cilichoroidal effusion-induced secondary angle closure glaucoma includes immediate discontinuation of topiramate, aqueous suppressants including oral or intravenous (IV) acetazolamide or IV mannitol, topical beta blockers, topical alpha-adernergics, topical carbonic anhydrase inhibitors, topical cycloplegics such as cyclopentolate or atropine and topical steroids.19 It is important to note that even though the carbonic anhydrase inhibitors are recognized for their potential sulfa cross reactivity, they are still included in the armamentarium of agents used to acutely reduce IOP in this pathology.19 The cycloplegics implement ciliary process relaxation deepening the anterior chamber. Atropine has the capability to stabilize the permeability of normal blood vessels, reducing fluid leakage. The steroids suppress cytokine and chemo-attractant formation (inflammation). Typically, the acute angle closure usually resolves within 24 hours to 48 hours and the induced myopia resolves within one week to two weeks of discontinuing the topiramate.19 If the condition is refractory to this initial approach oral or IV steroids can be attempted. In recalcitrant cases argon laser peripheral iridoplasty (ALPI) and other surgical interventions including choroidal drainage, vitrectomy, cataract extraction with intraocular lens placement and other glaucoma surgeries can be attempted.19,20
Argon laser peripheral iridoplasty (ALPI) is a simple and effective means of opening an appositionally closed angle in situations where laser iridotomy fails (pupil block is not the etiology) or when iridotomy cannot be performed.20 The procedure consists of placing burns (long duration, low power, and large spot size) in the extreme iris periphery (contraction spots) to contract the iris stroma between the site of the burn and the angle, physically/mechanically pulling open the angle.20
A number of additional conditions may lead to choroidal expansion and resultant secondary angle closure glaucoma without pupil block in eyes not at anatomical risk.16 These include complications from scleritis, Vogt-Koyanagi-Harada syndrome, complications from dense and extensive pan retinal photocoagulation, complications from retinal HIV infection and effects from cavernous sinus fistula.16
In this case the choroidal effusion was confirmed via the absence of pupil block (there was no iris Bombay, peripheral iridotomy had no effect) and observed with B-scan ultrasonography. The elevated intraocular pressure and choroidal effusion were managed by discontinuing the oral topiramte and beginning topical atropine 1 % BID, prednisolone acetate 1% QID, timolol maleate 0.5% BID and apraclonidine 0.5% BID. The IOP normalized in 24 hours returning to pre-topiramate levels measuring 14mm Hg, the visual acuity was reported as sharp and clear and the anterior segment was free from pathology.
| 1. Mitra A, Ramakrishnan R, Kader MA. Anterior segment optical coherence tomography documentation of a case of topiramate induced acute angle closure. Indian J Ophthalmol. 2014;62(5):619-22. 2. Yancey JR, Sheridan R, Koren KG. Chronic daily headache: diagnosis and management. Am Fam Physician. 2014;89(8):642-8. 3. Gupta PP, Thacker AK, Haider J, et al. Assessment of topiramate's efficacy and safety in epilepsy. J Neurosci Rural Pract. 2014;5(2):144-8. 4. Hurt RT, Edakkanambeth Varayil J, Ebbert JO. New pharmacological treatments for the management of obesity. Curr Gastroenterol Rep. 2014;16(6):394. 5. Fleming JW, McClendon KS, Riche DM. New obesity agents: lorcaserin and phentermine/topiramate. Ann Pharmacother. 2013;47(7-8):1007-16. 6. Liu L. Deconstructing the mechanisms of angle closure with anterior segment optical coherence tomography. Clin Experiment Ophthalmol. 2011;39(7):614-22. 7. Amini R, Whitcomb JE, Al-Qaisi MK, et al. The posterior location of the dilator muscle induces anterior iris bowing during dilation, even in the absence of pupillary block. Invest Ophthalmol Vis Sci. 2012;53(3):1188-94. 8. Liu J, Lamba T, Belyea DA. Peripheral laser iridoplasty opens angle in plateau iris by thinning the cross-sectional tissues. Clin Ophthalmol. 2013;7(9):1895-7. 9. SooHoo JR, Seibold LK, Kahook MY. Recent advances in the management of neovascular glaucoma. Semin Ophthalmol. 2013;28(3):165-72. 10. Debrouwere V1, Stalmans P, Van Calster J, et al. Outcomes of different management options for malignant glaucoma: a retrospective study. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):131-41. 11. Parikh R, Parikh S, Das S, Thomas R. Choroidal drainage in the management of acute angle closure after topiramate toxicity. J Glaucoma. 2007;16(8):691-3. 12. Obuchowska I, Mariak Z. Choroidal detachment--pathogenesis, etiology and clinical features. Klin Oczna. 2005;107(7-9):529-32. 13. Sellem E. Angle closure mechanisms of glaucoma. J Fr Ophtalmol. 2004;27(6 Pt 2):693-6. 14. Ikeda N, Ikeda T, Nagata, et al. Ciliochoroidal effusion syndrome induced by sulfa derivatives. Arch Ophthalmol 2002; 120(12):1775. 15. Rhee DJ1, Goldberg MJ, Parrish RK. Bilateral angle-closure glaucoma and ciliary body swelling from topiramate. Arch Ophthalmol. 2001;119(11):1721-3. 16. Sakai H, Morine-Shinjyo S, Shinzato M, et al. Uveal effusion in primary angle-closure glaucoma. Ophthalmology. 2005;112(3):413-9. 17. Congdon NG, Friedman DS. Angle-closure glaucoma: impact, etiology, diagnosis, and treatment. Curr Opin Ophthalmol. 2003;14(2):70-3. 18. Chen TC, Chao CW, Sorkin JA. Topiramate induced myopic shift and angle closure glaucoma. Br J Ophthalmol 2003;87:648-9. 19. Rapoport Y, Benegas N, Kuchtey RW, Joos KM. Acute myopia and angle closure glaucoma from topiramate in a seven-year-old: a case report and review of the literature.BMC Pediatr. 2014;14(4):96. 20. Ritch R, Tham CC, Lam DS. Argon laser peripheral iridoplasty (ALPI): an update. Surv Ophthalmol. 2007;52(3):279-88. |

