 |
A 76-year-old African-American female presented for decreased vision at distance and near without correction for the past two to three years. While her ocular history was unremarkable, her medical history was positive for diabetes over the past two years and hypertension for the past 20 years, which are both medically controlled with metformin and amlodipine, respectively. Per her report, her last fasting blood sugar level measured 125mg/dL with an A1c of 5.9% and her latest blood pressure reading measured 136/80. She denies any alcohol, smoking or illicit drug use.
On examination, her best-corrected visual acuities were 20/400 OD and 20/400 OS with a fluctuating prescription due to poor patient responses. Vision was slightly reduced to counting fingers at three feet in her right eye and 20/400 in her left during glare testing. Confrontation visual fields and extraocular motilities were full for both eyes. Pupils were equal, round and responsive to light with no afferent pupillary defect.
Intraocular pressures measured 21mm Hg for both eyes. Blood pressure measurement in office was measured to be 138/86.
Anterior segment health revealed 3-4+ nuclear sclerotic cataracts with brunesence in both eyes. A dilated fundus exam revealed changes (Figure 1). The left eye was completely normal. Optical coherence tomography (OCT) imaging of the right eye was also performed (Figures 2 and 3).
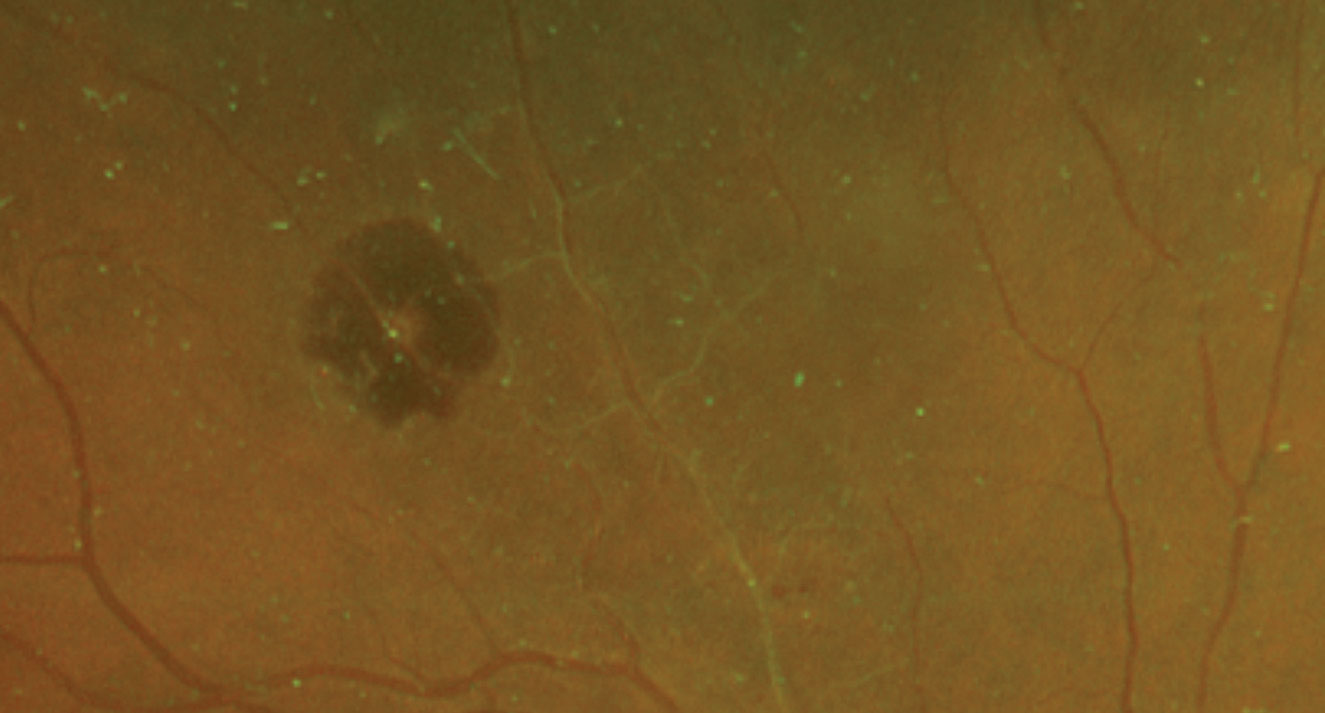 |
| Fig. 1. These dilated fundus shots show changes to the 76-year-old patient’s retina. Click image to enlarge. |
Take the Retina Quiz
1. Describe the fundus appearance of the right eye.
a. Attenuated vessels with an area of retinal pigment epithelium hypertrophy superiorly.
b. Hemorrhage superiorly underlying a vessel with an adjacent area of sclerotic vessels.
c. Choroidal neovascular membrane with surrounding subretinal hemorrhage superiorly.
d. Pigment clumping superiorly with an adjacent area of sclerotic vessels.
2. What is the likely diagnosis?
a. Diabetic retinopathy.
b. Branch retinal vein occlusion.
c. Exudative age-related macular degeneration.
d. Retinal arterial macroaneurysm.
3. What is the most common underlying cause?
a. Diabetes.
b. Hypertension.
c. Carotid occlusive disease.
d. Cardiac valve disease.
4. What is the appropriate treatment for our patient?
a. Pars plana vitrectomy.
b. Argon laser photocoagulation.
c. Observation.
d. Anti-VEGF injection.
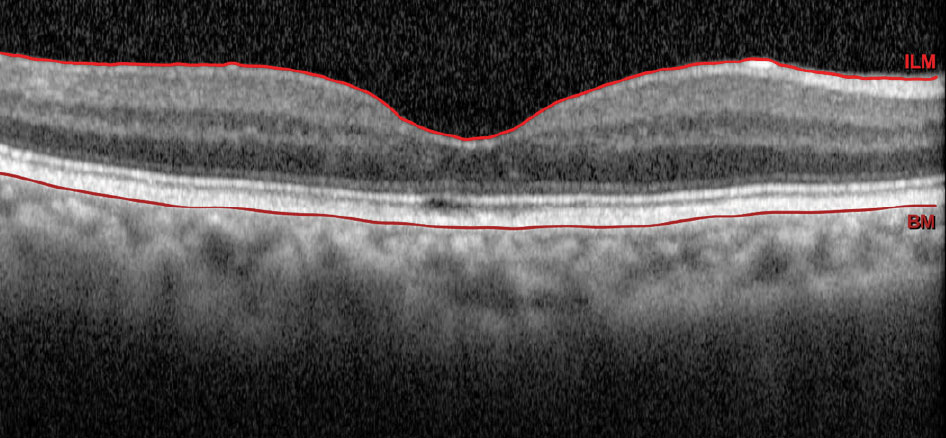 |
| Fig. 2. OCT of our patient’s right eye. Click image to enlarge. |
Diagnosis
Based on the history and clinical presentation, the patient was diagnosed with retinal arterial macroaneurysm (RAM) of the right eye. RAM is typically described as an idiopathic, acquired focal dilation of a major retinal arteriole that is typically located within the first three bifurcations, at branch points or at arteriovenous crossings.1 It is also most commonly reported to occur within the superotemporal quadrant of the posterior pole.2
In our patient, we see a whitish aneurismal dilation along the superior temporal artery with surrounding retinal hemorrhage. We also noted sheathing in several of the retinal arteries nasal to it, suggesting she had a previous vascular event, quite possibly an old branch retinal artery occlusion. No exudation or exudative retinal detachment was seen.
Discussion
Retinal arterial macroaneurysms present mainly in females in their sixth decade of life.1 It is typically a singular and unilateral finding, although 10% of cases reported bilateral RAMs and 20% of cases reported the presence of multiple RAMs along the same vessel or multiple vessels.2
The underlying pathophysiology of RAM is poorly understood. One hypothesis states that vessel wall fibrosis secondary to arteriosclerosis leads to decreased wall elasticity, that in combination with increased luminal pressure creates an aneurysmal dilation of the arteriole.3 Another hypothesis states that emboli cause damage to the endothelium of the vessel wall, rendering it weak and more likely for aneurysm formation.4 Yet other studies have claimed that prolonged hypertension can cause chronic venous stasis, which in turn predisposes arterioles to aneurysmal dilation.3 Regardless of the pathogenesis, the bottom line remains that a weakened vessel wall has a higher susceptibility to aneurysm formation.
RAM is rarely seen in practice. It often presents as focal dilations of a major arteriole structure that is either saccular or fusiform in shape. Saccular macroaneurysms (sac-like) balloon out on one side of the arteriole while fusiform macroaneurysms (round) balloon out on both sides of the blood vessel.4 Exudative RAM cases are thought to be fusiform in nature while hemorrhagic RAMs are thought to be saccular.4 The typical clinical course of a RAM involves enlargement of the arteriole, thrombosis, fibrosis and, ultimately, involution.1
RAMs can be categorized as exudative, hemorrhagic or quiescent. Exudative RAMs usually present with exudates found in a circinate pattern around the ruptured aneurysm with concomitant intraretinal and subretinal fluid accumulation.2 Hemorrhagic RAMs are larger than 1DD in area, can affect central vision and typically tend to leak blood into multiple layers of the retina.2 This is due to the fact that arteries are high flow vessels and when they rupture, they do so under significant pressure, pushing blood into multiple layers of the retina. As such, it is not uncommon to see blood in the subretinal, intraretinal, preretinal and vitreal layers of the eye.2 Fortunately, this is not the case in our patient. Our patient has more of a quiescent RAM, in which the hemorrhages or exudation spare the macula and do not affect final visual acuity.1
Many risk factors can predispose an individual to acquiring a RAM. Systemic hypertension is a major risk factor for the development of RAMs, accounting for 61% to 81% of cases.2 In fact, due to the presence of sclerotic vessels found adjacent to the RAM in our patient, hypertension is likely the predominating cause. Additional major risk factors include age (>60 years old) and gender (female).2 While not consistently observed in all cases, the literature states that conditions such as elevated lipid levels, diabetes, polyarteritis nodosa, sarcoidosis, rheumatoid arthritis and Raynaud’s phenomenon are linked to RAM.2 Differential diagnoses for this condition include, but are not limited to, diabetic retinopathy, radiation retinopathy, capillary hemangioma, Coats’ disease and wet macular degeneration.3 A thorough case history and various imaging modalities can be used to hone in on the diagnosis.
Imaging tools such as OCT of the macula, fundus photography, B-scan ultrasound and occasionally fluorescein angiography can be helpful in diagnosing retinal arterial macroaneurysms.2 While macular OCT can highlight the presence of edema and fundus photography can monitor the course of this condition, B-scan ultrasound is typically only used in cases when direct view to the fundus cannot be attained. In fluorescein angiography studies, RAM fills up uniformly in the early phase, thereby highlighting the focal dilation of the arteriole followed by a late leakage.4 For our patient, a baseline fundus photograph was attained to highlight the area of bleeding superotemporal to the macula with an adjacent area of sclerotic vessels.
Depending on the location and associated complications of a RAM, symptoms can vary greatly. Most patients are typically asymptomatic with RAM being an incidental finding during a dilated examination. Sudden, severe, painless vision loss associated with a RAM can be attributed to fluid or blood accumulation within the macula.1 Based on the superotemporal location of the RAM, our patient was asymptomatic. However, when vision loss does occur, it is not necessarily permanent. Vision restoration is usually achieved in these cases unless there is extensive hemorrhaging or chronic macular edema present.3 Ultimately, the location and severity of a retinal arterial macroaneurysm determines the initial and final visual outcome.
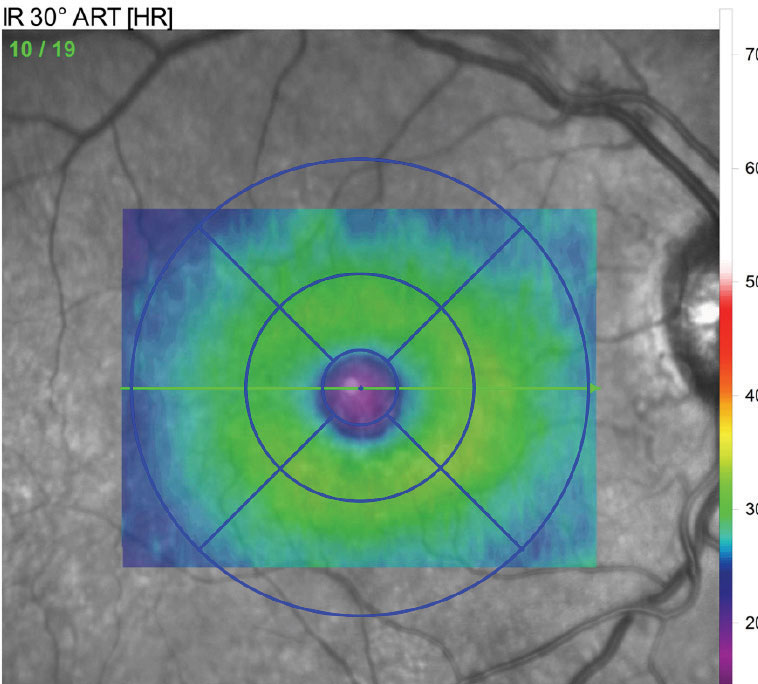 |
| Fig. 3. This retinal thickness map reveals crucial diagnostic information. |
Management
The natural history of this condition suggests that most RAMs involute spontaneously with a favorable visual outcome.4 As such, most patients can be safely observed at one to three month intervals until resolution.4 Indications for further treatment are determined based on the presence of exudates, fluid or blood within the macula as well as the persistent nature of this condition. Argon laser photocoagulation can be applied directly to the RAM to prevent it from rupturing.4 Indirect laser can also be used to decrease oxygen consumption and reduce blood flow to the RAM, thus lowering the amount of exudation from the surrounding capillaries.4 Anti-VEGF injections may also provide an alternative option for minimizing the presence of macular edema.3 Finally, in the setting of a vitreous hemorrhage, pars plana vitrectomy can be performed.2 Treatment varies on a case-by-case basis. Due to the peripheral location of the RAM, our patient is currently being monitored at monthly intervals.
Overall, RAMs are rare and mostly present as an incidental finding. As such, a keen eye is necessary to diagnose and treat this condition. While there are multiple treatment options available for RAMs, an established treatment protocol is yet to be determined.1 With that said, most RAMs run a benign course with a spontaneous regression and a favorable final visual outcome.
Dr. Jayasimha is an optometric resident at Bascom Palmer Eye Institute in Miami.
1. Feldman B, Mozayan E, Karth P, Sjaj V. Macroaneurysm. EyeWiki. eyewiki.aao.org/Macroaneurysm. October 17, 2015. Accessed March 8, 2019 2. Venkateswaran N, Flynn H, Leung M. Managing retinal macroaneurysms. Rev Ophthalmol. 2018;25(11):50-3. 3. Ng W, Mathur R, Ting D. Diagnosis and management of retinal arterial macroaneurysm. Eyenet. www.aao.org/eyenet/article/diagnosis-of-retinal-arterial-macroaneurysm. June 2018. Accessed March 8, 2019. 4. Speilburg A, Klemencic S. Ruptured retinal arterial macroaneurysm: Diagnosis and management. J Optometry. 2014;7(3)111-76. |

