Neurological insults can affect visual processing without creating loss of acuity. In other words, patients test 20/20, but something else is keeping them from seeing properly. These patients report to the eye clinic with puzzling symptoms. Their insults develop following stroke (ischemic or hemorrhagic), neurodegenerative diseases, trauma, infection, carbon monoxide exposure, toxic exposure to other chemicals, demyelinating disease, intracranial mass or psychiatric illness.
The patient may be unable to articulate the problems they are experiencing or, worse yet, they may be unaware of any issue. Newly detected abnormalities include visual field loss (which can sometimes manifest as neglect of one side), or difficulty navigating, following directions, identifying location where one is, naming objects or people, telling time, recognizing faces or one’s self in the mirror, reading, writing, seeing patterns or doing simple arithmetic.
All of these issues can be produced by neurologic disease. Yet, optometrists may misunderstand the complaint and misdiagnose the underlying issue.
Here, we review how strokes and other neurological conditions are likely to appear in your exam chair and how you can properly comanage these patients’ systemic health while taking a lead role in safeguarding their visual health.
 |
Sharing with patients this vegetable man image is a method by which clinicians can determine if patients have a particular issue with their visual processing. Patients with simultagnosia, for example, can describe the fruits and vegetables but won’t recognize that they form the shape of a man. Click image to enlarge. |
The Clock-Drawing Test
Take this case, for example. A 65-year-old female was referred to our retinal service by an outside practitioner who could not remedy a reading symptom she described as “distortion of the page.” The practitioner documented no ocular etiologies. They included records of testing that demonstrated 20/25 visual acuity (VA) at distance and near with a +0.50/+2.50 sphere refraction OU.
The data of the exam revealed no change in distance or near refraction, no appreciable change to her crystalline lenses, normal topography (no evidence of keratoconus), normal Amsler grid, no afferent pupillary defect (APD), no color or brightness abnormality, normal fundus appearance, normal central screening visual field and normal optical coherence tomography (OCT) (5-line raster, macula). The retina specialist completed a dilated exam that confirmed these findings. A call was placed to her general practitioner, who explained that she had a history of right-sided parietal lobe cerebrovascular accident (CVA) from three years earlier, and her symptoms began after that. With this information, we decided to perform a “clock-drawing test,” in which the patient is presented with a blank circle and asked to fill in the clock hours. The filled-in clock face demonstrated initial asymmetric distribution of the clock hours skewed to the right with subsequent correction. This is indicative of mild left hemi-spatial neglect. Visual field testing was not conclusive for hemianopia but demonstrated global loss of sensitivity. It was then we realized that the medical team and ophthalmic teams were independently managing her symptoms without ever associating them to the location of the CVA.
Her condition was re-explained to her and she was referred to low vision services, where she received vision rehabilitation care. She was provided with reading aids including a typoscope, electronic magnifiers and an illuminated hand-held magnifier to help with her work (jewelry crafting).
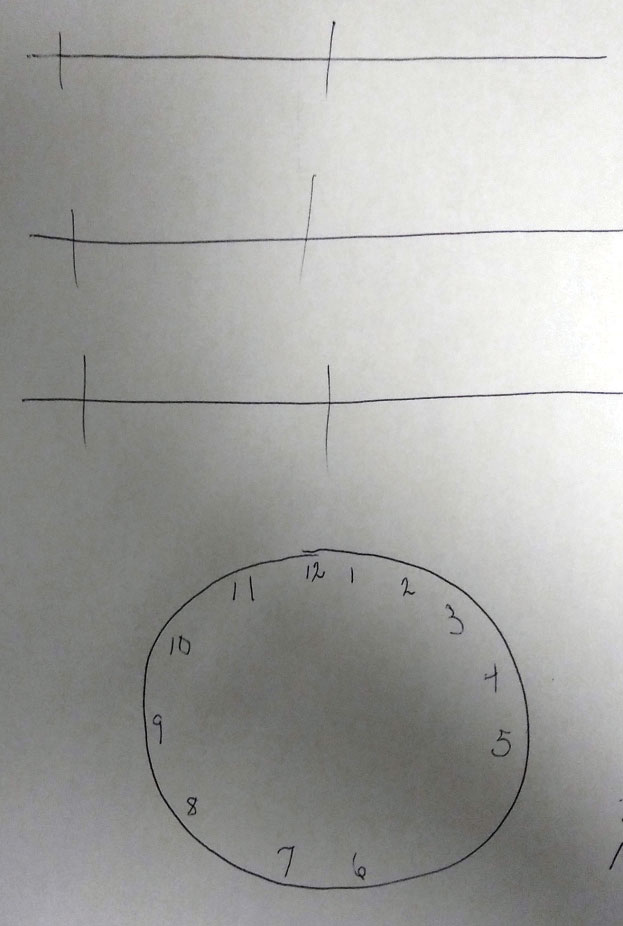 |
| Tasks like this clock-drawing test can help determine the extent to which an injury has impacted the patient’s brain. Click image to enlarge. |
For Each Behavior, An Anatomy
Stroke symptoms-—even visual symptoms—can be missed by any doctor, and that includes visual symptoms. When a primary vascular pathology produces changes in personality or mentation in the absence of a motor deficit (cranial nerve III, IV, VI, VII palsy, hemiparesis or hemiplegia) the possibility of illness or disease may be ignored or rationalized as “a bad mood or day” by the patient, friends, family and co-workers. This leads to a disassociation of the signs from symptoms, leading to a lack of testing.
Every behavior has an anatomy. Architecturally, the brain is compartmentalized. If the area handling a task is affected, the system becomes dysfunctional.
The retina is designed to convert the electromagnetic energy of photons (the particles of light) into electric impulses and distribute it into the brain via the visual pathway.
The visual pathway carries electric impulses to three places: the Edinger-Westphal nucleus in the midbrain (pupillary constriction pathway—cholinergic), the occipital lobe (visual development center) and the pulvinar/sympathetic pathway (pupillary dilation pathway—adrenergic).1,2
Defects in any pathway can produce a problem specific to that pathway; a problem in the pupillary constriction pathway will produce constrictive abnormalities (a pupil that fails to constrict such as an APD, Adie’s tonic pupil and Argyll Robertson pupils). Problems in the three neuron arc of the pupillary dilation pathway will produce abnormalities of dilation (e.g., Horner’s syndrome), and issues with the traditional pathway carrying impulses to the occipital lobe will produce visual field defects and variable visual loss.1
Once the occipital lobe has created an image, the information enters the ventral stream. This is where understanding and context begins. When associative centers of the brain are either damaged or denied their neural input, seeing continues in the absence of understanding. This is called an agnosia.
The term “visual agnosia” refers to difficulty naming or identifying visual targets. The pathology occurs in the context of an intact visual pathway, intact memory and intact ability to think. Visual agnosia can be further divided into apperceptive agnosia (AA), or the impairment of processing patterns, and associative agnosia (AsA), or the impairment of visual memories and their meanings.3
Apperceptive Agnosia
This visual impairment results in a patient’s inability to identify patterns; as such, they have difficulty identifying objects because they cannot see the way components form a single image. When shown a simple object, such as a mug or cup, these patients cannot draw it. An AA patient does not suffer from visual scotoma or lost acuity.
AA is the result of pathology that interrupts the ventral stream (connections between the occipital lobe and temporal lobes) in the occipital and temporal association areas in the distribution of the middle cerebral artery (the most common location of CVA.1,4
AA patients are able to name the attributes of an object but are not able to identify the whole object. They maintain their ability to describe objects in detail and then recognize the object (name and function) when the object is placed in their hand.3,5 As an example, an AA patient would not be able to draw or identify a set of car keys. They would be able to look at the set of car keys and describe what it is made of or describe the jagged edges of the “teeth” of the keys. However, when the set of keys were placed into their hand they are able to both name the object (a set of car keys) and describe what they do. These individuals also fail the Gollin form test.3,6 When presented with Gollin forms, the individual sees a conglomeration of shapes but not the pattern they create. The AA patient looking at the toothpick-like illustration of the word “THIS” would report seeing the number “7415.”7
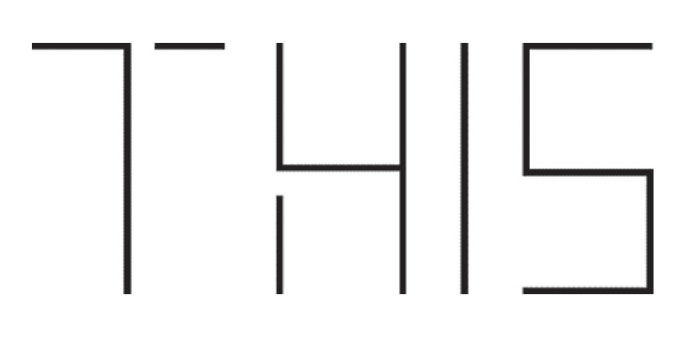 |
A suspect for appreceptive agnosia can be offered this Gollin form test. Those with the condition see the shapes, but not the pattern they create. For instance, above, they would report seeing the number 7415, but not the word “THIS.”3 Click image to enlarge. |
Associative Agnosia
This is often “category-specific” to the portion of the brain that has been affected. Here, the damaged area of the brain is the occipital-temporal region.1,3
Damage here causes patients to lose the ability to identify category-specific visual information. For example, the patient may be unable to identify a dog by seeing it but can recognize a dog by its bark.8 Unlike AA patients, they can draw a reasonable likeness of these objects when they are shown them but cannot explain what they are. However, when asked what a “lion” or “car” or “chair” is, they can articulate the correct answer.
Global agnosia occurs in the worst cases. Here, diffuse hypoxic injuries such as carbon monoxide poisoning create widespread damage over large cerebral expanses involving visual memories and their associations.3,5
Prosopagnosia
This is an inability to recognize faces and is associated with focal temporal-occipital junction lesions.3,5,9,10
In the apperceptive form of prosopagnosia (PA), patients experience difficulty discriminating specific features of a face. In the associative form, the patient can identify, compare and match features of a face but can’t match it to the person they know.3 An extreme example is “mirror agnosia,” a form of prosopagnosia where the subject is unable to recognize themselves in the mirror.11,12
Clinical tests used to uncover PA include the “famous faces test” and the “Benton facial recognition test.”3,9
It is likely that many PA patients have broader object agnosias not limited to faces, but the inability to recognize a face stands out due to social ramifications.9
Interestingly, people can be born with prosopagnosia (2.5% of the general population).3 These people appear to have difficulty remembering if they previously met someone and also appear to have trouble with people’s names.3
Simultagnosia
Lesions in the dorsal stream and the inability to process multiple details of an object at the same time—a sort of narrowed window of attention—are indicative of simultagnosia (SA). Unlike those with other agnosias, these patients are often symptomatic to others; they appear as inattentive or aloof.3
The “cookie theft picture” (shown on p. 80) is a classic test for SA.1 Here, the SA patient verbalizes that the dishes are being cleaned but does not notice the water overflowing from the sink or the child stealing cookies. These patients are often labeled as clumsy because they have difficulty navigating around obstacles in a room. They also fail the “vegetable man test” (shown on p. 75), where they’re asked to view the picture of a man whose image is constructed of fruits and vegetables. The SA patient will describe individual fruits and vegetables but will fail to see the person.13 Other tests include the “alphabet test” (see p. 82), where a letter of the alphabet is created by other, smaller letters.14
SA is common in occipital lobe stroke, although it can also result from neurodegenerative diseases such as posterior cortical atrophy.3 It may also occur in the context of Bálint syndrome (acquired triad of simultagnosia, optic ataxia with spatial distortion and ocular motor apraxia) secondary to parietal lobe stroke.15 SA is a diagnosis that can be predicted based upon neuroimaging; however, cognitive dysfunction, hemineglect and visual field defects should be ruled out before administering testing.
Topographic Agnosia
Patients who have difficulty navigating familiar environments or get lost in their own home may be experiencing topographic agnosia (TA).3
These patients can remember and verbally repeat directions and can explain how to get from one place to the other but are unable to execute the task.3 Difficulty with navigation is reported in up to 30% of post-stroke patients and has a strong correlation with subjective quality of life.16 Again, this occurs in the context of intact cognition and memory.3 There are two possible types. The first, “landmark agnosia,” occurs when the patient has difficulty recognizing significant landmarks that they encounter in their environment.3 It can affect both novel and familiar landmarks.17 Researchers believe this occurs from damage to the lingual and fusiform gyri.17 The second type is the inability to construct a mental spatial map and arises from damage to the hippocampus or retrosplenial cortex.3
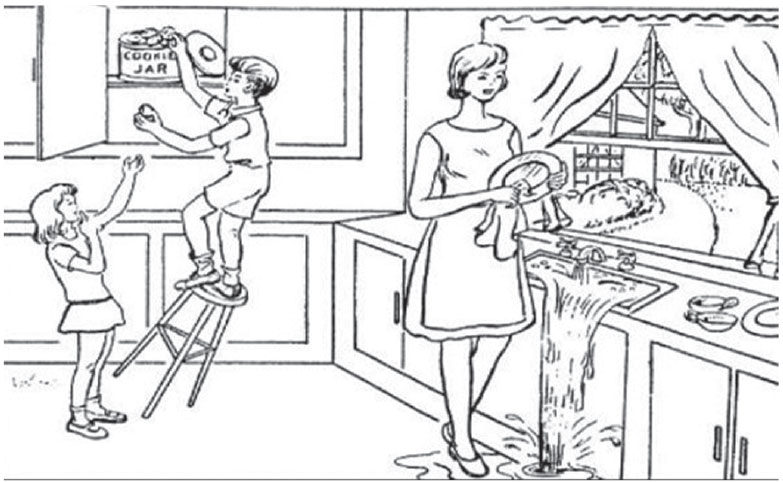 |
| The “cookie theft” test for SA uses this picture. Patients with the condition will verbalizes that the dishes are being cleaned but will not notice the water overflowing from the sink or the falling child stealing cookies. Click to enlarge image. |
Reading, Writing, Arithmetic
The inability to read, with and without agraphia (the inability to write) is an acquired “form-agnosia” preventing reading in previously literate patients and is called alexia.3,18-21 The issue is secondary to damage in the area of the brain that completes cortical processing of language.20 The lesion is in the left occipital lobe and the splenium of the corpus callosum. These patients experience an absence of perceptual or language deficits.
Pure agraphia is rare. It is more often accompanied by alexia, or aphasia (inability to articulate). Individuals with apraxic agraphia can spell normally when asked to complete the task orally, in the setting of otherwise intact visual/language processing.21 The cortical area responsible for creating apraxic agraphia is unclear as lesions in the parietal, frontal or thalamic areas can all cause agraphia.21
Acalculia is the acquired impairment of arithmetic skills despite normal reasoning and language skills.22,23 These patients understand what a number is and can see numbers on a page but are unable to perform the reasoning skills used in basic arithmetic. It is associated with left posterior parietal lobe damage.22
Visual Orientation
Allochiria is when a patient responds to stimuli presented to one side of their body as if the stimuli had been presented on the opposite side.24,25 Allochiria typically occurs following left hemispheric stroke.26 The condition results in mental reversal of hemispheric orientation. In the clock-drawing test, a patient with allochiria can correctly copy the 9 o’clock number onto a blank clock face but when asked to draw a clock face from memory, they will transpose it to the wrong side (the 3 will wind up in the 9 position).
This is in contrast to a patient with hemispatial neglect, who will draw most clock hours in the preserved hemifield.
Peripheral visual awareness plays a role in seeing and spatial awareness. It permits focus of attention and aids in directing accurate motor commands toward objects. Loss of the visual field creates a lack of awareness and ability to interact with objects in space.27 Negative scotomata (neural field loss) are missing areas in the visual field filled in by the brain with no perception of missing vision. Patients with this issue often complain of vertigo (dizziness), balance difficulty and reading difficulty.27
Hemifield visual field loss is a common sequela of stroke, space-occupying lesion or traumatic brain injury.28 Estimates of its prevalence in stroke range from 10% to 57% of patients.28,29 Unfortunately, some patients either do not realize their field loss exists or cannot articulate the experience secondary to cognitive decline or inability to communicate.30,31 Awareness often arises when patients fail to perform tasks that require visual feedback, such as navigating an unfamiliar space, driving or reading.28,30
Automated visual field testing can help discover hemifield deficits, but are inconsistent.28 Confrontation visual fields are less sensitive and can miss up to 50% of severe defects.28,30 Clinicians must be good observers. Tell-tale exam room behaviors—such as scuffs and scrapes on one side of the patient’s clothing or shoes, exaggerated head turning toward the affected side or persistently reading one side of the line of letters on the visual acuity chart or reading material—may all serve as clues to missing visual fields.
Visual neglect is often associated with hemifield visual field loss and occurs when the patient lacks awareness of the contralesional visual space. It most commonly occurs on the left side, as the left cortical hemisphere monitors only the right hemispace and the right cortical hemisphere monitors both sides.5 A variant form called allocentric object-based neglect refers to the symptom of ignoring the contralesional side of objects, regardless of the object’s egocentric position.25
 |
| The SA patient will look at this image and see the smaller letters Es, but not the larger A they make up. Click image to enlarge. |
The OD’s Role
Management of agnosias and other suspected neurological defects depends on how recently it started. If symptoms present acutely (within 48 hours) the patient must be rapidly transported to a stroke center for evaluation and intervention with thrombolytic agents (most effective within 4.5 hours of ischemic onset).32
Symptoms that have been present for 48 hours to two weeks should still be managed with urgency; these patients require evaluation and neuroimaging but this can be best accomplished through coordination with a primary care physician (PCP).33 A phone call should be placed to the PCP immediately, ideally for same-day evaluation. The PCP may elect to have the patient evaluated in the emergency department or as an outpatient.
Mitigation of diabetic and hypertensive risk factors as well as initiation of aspirin or statin drugs will likely be initiated by the PCP within 24 hours.33 More often, symptoms are vague and chronic when discovered and require investigation to uncover the nature of the problem along with its underlying cause. After stroke, the most common causes of adult-onset neurological disorders are Alzheimer’s disease and demyelinating disease.34
The role of the optometrist is to refer to neurology, communicating that all ocular findings are normal (unless visual fields are affected) and that the symptoms require further investigation. The OD also plays a valuable role in providing rehabilitative and low vision services along with education on the nature of their visual perception disorder.
Dr. Karbach is a clinical instructor at The Eye Institute, Pennsylvania College of Optometry at Salus University.
Dr. Gurwood is a professor at Pennsylvania College of Optometry at Salus University.
| 1. Girkin CA, Miller NR. Central disorders of vision in humans. Surv Ophthalmol. 2001;45(5):379-405. 2. Geschwind N. The borderland of neurology and psychiatry: some common misconceptions. In: Benson DF, Blumer D, eds. Psychiatric Aspects of Neurologic Disease. Vol 1. New York: Grune and Stratton; 1975. 3. Haque S, Vaphiades MS, Lueck CJ. The visual agnosias and related disorders. J Neuroophthalmol. 2018;38(3):379-92. 4. Hedna VS, Bodhit AN, Ansari S, et al. Hemispheric differences in ischemic stroke: is left-hemisphere stroke more common? J Clin Neurol. 2013;9(2):97–102. 5. Greene J. Apraxia, agnosias, and higher visual function abnormalities. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:v25-v34. 6. Chikhman V, Shelepin Y, Foreman N, et al. Incomplete figure perception and invisible masking. Perception. 2006;35(11):1441-57. 7. Landis T, Graves R, Benson DF, Hebben N. Visual recognition through kinaesthetic mediation. Psychol Med. 1982;12(3):515-31. 8. Devinsky O, Farah MJ, Barr WB. Chapter 21 Visual agnosia. Handb Clin Neurol. 2008;88:417-27. 9. De Renzi E. Disorders of visual recognition. Semin Neurol. 2000;20(4):479-85. 10. Corrow SL, Dalrymple KA, Barton JJ. Prosopagnosia: current perspectives. Eye Brain. 2016;8(9):165-75. 11. Chandra SR, Issac TG. Mirror image agnosia. Indian J Psychol Med. 2014;36(4):400-3. 12. McNeil JE, Warrington EK. Prosopagnosia: a face-specific disorder. Q J Exp Psychol A. 1993;46(1):1-10. 13. Dalrymple KA, Barton JJ, Kingstone A. A world unglued: simultanagnosia as a spatial restriction of attention. Front Hum Neurosci. 2013;7:145. 14. Brazis PW, Graff-Radford NR, Newman NJ, Lee AG. Ishihara color plates as a test for simultanagnosia. Am J Ophthalmol. 1998;126(6):850-1. 15. Amalnath SD, Kumar S, Deepanjali S, Dutta TK. Balint syndrome. Ann Indian Acad Neurol. 2014;17(1):10–11. 16. van der Ham IJM, Martens MAG, Claessen MHG, van den Berg E. Landmark agnosia: evaluating the definition of landmark-based navigation impairment. Archi Clin Neuropsych. 2017;32(4):472-82. 17. Takahashi N, Kawamura M. Pure topographical disorientation--the anatomical basis of landmark agnosia. Cortex. 2002;38(5):717-25. 18. Mendez MF, Cherrier MM. Agnosia for scenes in topographagnosia. Neuropsychologia. 2003;41(10):1387-95. 19. Rivest J, Svoboda E, McCarthy J, Moscovitch M. A case study of topographical disorientation: behavioural intervention for achieving independent navigation. Neuropsychol Rehabil. 2018;28(5):797-817. 20. Starrfelt R, Shallice T. What’s in a name? The characterization of pure alexia. Cogn Neuropsychol. 2014;31(5-6):367-77. 21. Rupareliya C, Naqvi S, Hejazi S. Alexia without agraphia: a rare entity. Cureus. 2017;9(6):e1304. 22. Ardila A, Rosselli M. Acalculia and dyscalculia. Neuropsychol Rev. 2002;12(4):179-231. 23. Ardila A, Rosselli M. Cognitive Rehabilitation of Acquired Calculation Disturbances. Behav Neurol. 2019;2019:3151092. 24. Gnanapavan S, Jaunmuktane Z, Baruteau KP, et al. A rare presentation of atypical demyelination: tumefactive multiple sclerosis causing Gerstmann’s syndrome. BMC Neurol. 2014;14(4):68. 25. Rorden C, Hjaltason H, Fillmore P, et al. Allocentric neglect strongly associated with egocentric neglect. Neuropsychologia. 2012;50(6):1151-7. 26. Lepore M, Conson M, Grossi D, Trojano L. On the different mechanisms of spatial transpositions: a case of representational allochiria in clock drawing. Neuropsychologia. 2003;41(10):1290-5. 27. Khan S, Leung E, Jay WM. Stroke and visual rehabilitation. Top Stroke Rehabil. 2008;15(1):27-36. 28. Goodwin D. Homonymous hemianopia: challenges and solutions. Clin Ophthalmol. 2014;8(9):1919-27. 29. Pollock A, Hazelton C, Henderson CA, et al. Interventions for visual field defects in patients with stroke. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD008388. 30. Ghannam ASB, Subramanian PS. Neuro-ophthalmic manifestations of cerebrovascular accidents. Curr Opin Ophthalmol. 2017 Nov;28(6):564-572. 31. Rowe FJ, Wright D, Brand D, et al. A prospective profile of visual field loss following stroke: prevalence, type, rehabilitation, and outcome. Biomed Res Int. 2013;2013:719096. 32. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317. 33. Bernheisel CR, Schlaudecker JD, Leopold K. Subacute management of ischemic stroke. Am Fam Physician. 2011;84(12):1383-8. 34. World Health Organization. Neurological Disorders public health challenges. www.who.int/mental_health/neurology/neurological_disorders_report_web.pdf. March 2006. Accessed July 29, 2019. 35. Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination. 3rd ed. Boston: Pearson; 2000. |

