Many optometrists avoid diagnosing and managing patients with neuro-ophthalmic disorders. We may perceive these cases to be very complex, time-consuming and possibly vision- or life-threatening.
While challenging, these conditions can be successfully managed by optometrists and comanaged with other specialties.
This article reviews each element of the exam, along with the appropriate ancillary testing required for proper diagnosis. Our goals are to give a good clinical approach for diagnosis and management, to help you decide which cases require non-urgent, urgent or emergent attention, and to encourage you to comanage certain disorders with neurology, neuro-surgery and any other appropriate specialty.
(Starting in the March 2015 issue, we’ll also be presenting neuro-ophthalmic case reports and discussions every other month.)
|
|
|
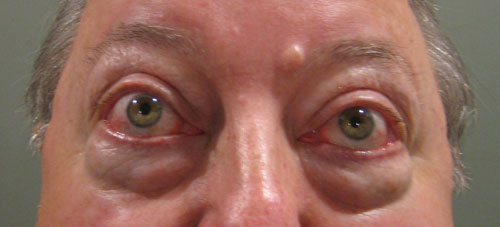
| This patient with thyroid orbitopathy has moderate proptosis and periorbital edema. He also has significantly reduced extraocular motility. |
History
By taking a detailed history, the practitioner can start making a list of differential diagnoses, and then direct the exam toward narrowing that list to find the etiology. This approach helps to keep clinical testing specific to the problem, instead of performing unnecessary tests.
Here are some of the more common neurologic complaints optometrists encounter:
• Diplopia. Your first question about double vision should be whether it’s monocular or binocular.
Monocular diplopia may be a result of uncorrected refractive error, keratoconus, cataract or maculopathy. Polyopia, which can occur following cerebral damage, is a perception of two or multiple images that can be seen monocularly.1 However, this is a very rare complaint and patients will usually have a prior neurologic cause such as stroke or traumatic brain injury.
In patients with true binocular diplopia, horizontal diplopia is frequently a result of a sixth nerve palsy or internuclear ophthalmoplegia (INO), while vertical diplopia is usually from a fourth or third nerve palsy. The diplopia is more prominent when the patient looks toward the affected muscle. For example, a patient with a left sixth nerve palsy will complain of horizontal diplopia that worsens upon left gaze.
Decompensating phorias can be either horizontal or vertical, but are usually intermittent rather than constant, as seen with nerve palsies and INO. Diplopia from thyroid orbitopathy can also be horizontal or vertical, but is typically present with other signs and symptoms, such as pain, pressure, proptosis, periorbital edema and chemosis/injection. Myasthenia gravis (MG) should always be a differential diagnosis, especially if the diplopia is variable, not consistent with any of the nerve palsies or if there are other symptoms such as lid ptosis, fatigue, dysphagia or dyspnea.
• Vision loss/disturbances. Noting whether the vision loss is monocular or binocular, along with its frequency, can be very helpful in narrowing down potential causes. Transient monocular vision loss, or amaurosis fugax, may be a precursor to both ocular and systemic pathology. A retinal embolus or artery occlusion, carotid artery insufficiency, non-arteritic ischemic optic neuropathy (NAION), arteritic ischemic optic neuropathy (AION) or vein occlusion may present with warning signs of fleeting vision loss. Alternatively, individuals who experience sudden binocular vision loss are more likely to have intracranial pathology.
Documenting the frequency of vision loss is also helpful in determining the cause. Loss of vision lasting a few seconds can point to papilledema or an impending AION.2 Retinal emboli and transient ischemic attacks (TIAs) typically cause vision loss for seconds to minutes, while visual phenomena from non-neurologic type migraine may last for hours, but typically less than 24 hours.
• Headaches. Headaches are a very common complaint and often not a result of serious pathology. However, certain symptoms or characteristics may imply vision- or life-threatening causes. Headaches that are newly noted, have different severity, frequency or duration––as well as those noted upon waking, those that wake the patient during the night or those that are unresponsive to pain medication or accompany other neurologic symptoms––should all be explored.3
A sudden-onset, severe headache—typically reported as “the worst headache of my life”—can be due to an intracranial hemorrhage or carotid artery dissection, and should be evaluated emergently.3 Neck pain and swelling or a Horner’s syndrome may be noted on the same side of the headache in carotid artery dissection patients.
Be sure to rule out giant cell arteritis (GCA) in individuals older than 55 years who experience headache accompanied by symptoms such as jaw claudication, scalp tenderness, fatigue, weight loss and generalized weakness.
Headaches that are worse in the morning, exacerbated by changes in posture and accompanied with transient visual obscurations, nausea, vomiting and tinnitus are typical for increased intracranial pressure.
Painful, monocular vision loss is characteristic of optic neuritis. The pain is usually worse with eye movement. Multiple neurological symptoms—such as paresthesias, ataxia, diplopia, fatigue, Uhthoff’s phenomenon and Lhermitte’s sign—may be present in patients with optic neuritis associated with multiple sclerosis.
|
|
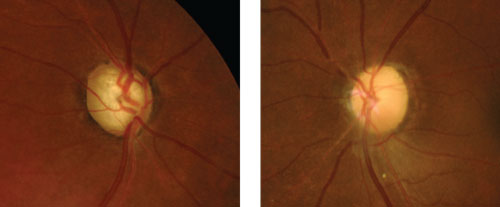
| Compare glaucomatous optic nerve pallor from total cupping (left) to non-glaucomatous pallor from optic atrophy due to an old ischemic optic neuropathy (right). |
|
 |
|
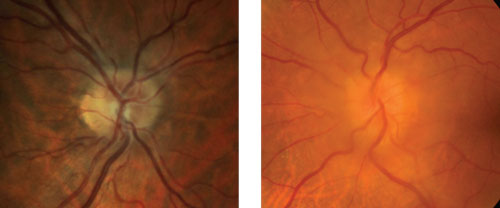
| Which is true disc edema? At left is pseudo disc edema from crowding of the optic disc. At right is true disc edema in a patient with a sphenoid wing meningioma. |
|
External Exam
• Gross observation.
Facial and lid abnormalities can be indicators of neurologic eye disease. These include signs of ptosis, proptosis, resistance to retropulsion, lagophthalmos, facial weakness, head turn or tilt, and blepharospasm.
It can be helpful to observe the patient in the waiting room (i.e., if they are turning or tilting their head, favoring one eye, showing difficulty with reading or watching television). This is especially beneficial with children who may not be able to give accurate histories or patients who are malingering.
• Extraocular motility. Perform extraocular motilities to look for any restricted gaze or pursuit deficits. Saccades can also be tested to help localize an issue. For example, slow saccades can be noted with a neurogenic process vs. a restrictive process, which will have normal saccades.4 Finally, the eyes should also be examined for nystagmus.
• Confrontation field testing. While automated perimetry is the standard of care, don’t skip confrontation testing, which can quickly screen for certain key field defects. For instance, we’ve examined asymptomatic patients with no other exam findings except for confrontation field defects, which led to diagnoses of pituitary adenomas and prior cerebrovascular accidents (CVA). Although confrontation field testing has a fairly low sensitivity for arcuate defects and bitemporal hemianopsia, it has a high sensitivity for detection of altitudinal defects and homonomous hemianopsia.5,6
• Pupillary testing. This is a great objective measurement for identifying neuro-ophthalmic conditions. Pupil size should be measured in both dark and bright illumination to help differentiate a sympathetic vs. parasympathetic issue. For example, Horner’s syndrome, which is an oculosympathetic palsy, will have anisocoria greater in dim illumination, while a third nerve palsy will have anisocoria greater in bright illumination. Afferent pupillary defects will be present with optic nerve and retinal disorders such as NAION, AION, optic neuritis and artery occlusions. In patients with an Adie’s tonic pupil, the pupil generally has an irregular shape and a slow constriction, often with sectoral paralysis. It will constrict with pilocarpine 0.125%, however.
• Cover test. This test is critical in evaluating patients with diplopia. If there is a small deviation, testing extraocular motilities is not sufficient for diagnosing a fourth or sixth nerve palsy. The cover test needs to be measured in all views of gaze in order to localize most deviations. We find the easiest method to measure the deviation is to use a prism bar during cover test while the patient is fixating at a distant target.
Anterior Segment Exam
While the majority of neuro-ophthalmic findings are localized to the posterior segment of the eye, a number of pertinent findings can manifest within the anterior structures. For instance, unilateral corkscrew vessels of the conjunctiva can be an indication of intracranial dural arteriovenous malformation (AVM) or carotid cavernous fistula (CCF). The phakomatoses—a group of congenital disorders characterized by systemic hamartosis—can manifest as hyperpigmented lesions of the iris, known as Lisch nodules.7
In addition, anterior chamber cell and flare in an elderly patient may be an indication of ocular ischemia secondary to carotid occlusive disease.
Funduscopy
Posterior segment findings are best observed with a combination of viewing modalities including direct, indirect and non-contact high-resolution imaging.
In the setting of optic nerve pathology, the optic nerve has only two responses to injury: atrophy or edema, whereby the former is the end result of any pathologic process. Some of the more common causes of pathology include compression, ischemia, inflammation, infiltration and metabolic or toxic disturbances. Sometimes, congenital anomalies of the optic nerve may simulate pathologic processes.
The color of the optic disc depends on light reflecting off the surface vessels and nerve fiber layer (NFL). In cases of pseudophakia and high myopia, a healthy disc may appear pale. However, in cases of true optic neuropathy, the clinical examination may yield isolated or combined pathological processes such as NFL dropout, loss of peripapillary capillaries, visual acuity and field defects, as well as an afferent pupillary defect.8,9
Individuals with swelling of the optic nerve often present as a diagnostic challenge. Most cases can be categorized based on timing of the event, visual acuity, laterality, associated systemic symptoms, and appearance of the optic nerve and retinal vasculature. Older individuals who present with painless vision loss, optic nerve edema and systemic vascular disease are at risk for NAION. Accordingly, the optic nerve may show sectoral edema with dilated capillaries and an altitudinal visual field defect. Nonetheless, immediate complete blood count (CBC) with platelets, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) must be ordered to discount arteritic disease even when constitutional symptoms of temporal arteritis are absent. While the two may be indistinguishable from a clinical appearance, some have described pallid swelling of the disc and worse visual acuity when discussing AION.9
Younger individuals who present with insidious vision loss in one eye and pain on eye movement with either normal or hyperemic swelling of the disc may be suffering from an optic neuritis event or a space-occupying intraorbital process that occurs with transient vision loss in peripheral gaze positions. Subsequent testing of the visual field, color vision, afferent system and NFL will allow for quantifying the pathology. In cases of bilateral disc edema, it is critical to assess the appearance of the optic nerve with respect to its rim tissue, NFL, vasculature and presence or absence of venous pulsation. Bilateral opacification of the nerve fiber layer, splinter hemorrhages at or adjacent to the disc, and lack of spontaneous venous pulsation are highly suggestive of increased intracranial pressure, which warrants emergent neuroimaging to discount an intracranial hemorrhage or mass. In the event the imaging is unremarkable, then lumbar puncture can be scheduled to measure the opening pressure and evaluate for idiopathic intracranial hypertension.
Lastly, a small physiologic cup with normal coloring, anomalous vasculature branching (i.e., trifurcations), disc drusen, absence of a high watermark sign and no frank opacification of the NFL is more likely to represent pseudopapilledema.
However, in most cases, determining true edema from congenital disc anomalies cannot be made by clinical exam alone. Ancillary testing of the visual field, B-scan ultrasound, optic coherence tomography (OCT) and serial photography of the optic disc will allow for both structural and functional assessment.
Visual Field Testing
In neuro-ophthalmic disease, visual field assessment is important for evaluating lesions thought to be affecting the visual pathway, as well as for monitoring progression of optic nerve disease and intracranial pathology. It is most commonly used in guiding treatment and monitoring for conditions such as idiopathic intracranial hypertension (IIH), optic neuropathy, pituitary adenomas and other sellar lesions.10
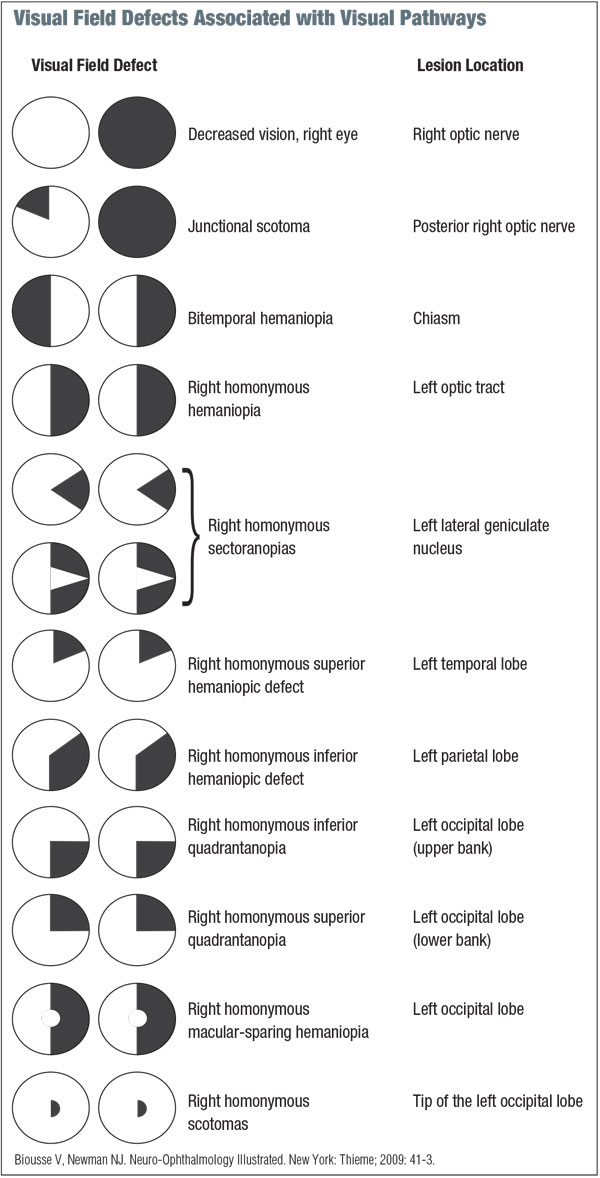
While there are diverse applications, visual field testing essentially serves two main functions: localization of the visual pathway and assessment for progression or regression analysis. (See “Visual Field Defects Associated with Visual Pathways.”)
|
Additionally, testing can be performed by a number of different techniques, including confrontation (with finger counting, red target or facial Amsler), tangent screen, Goldmann kinetic perimetry and standard automated perimetry (SAP). The confrontation technique makes up the majority of our screening fields and is reliable and efficient. When compared to Goldmann and automated perimetry, the confrontation method has a sensitivity of approximately 20% for arcuate, 50% for bitemporal, 70% for homonymous hemianopia and nearly 100% for altitudinal defects.5,6
Remember that while a defect identified by confrontation field testing has a relatively high predictive value of true defects confirmed with formal perimetry, confrontation testing has many limitations. Patients in whom there is a history of neurologic deficit, certain or suspected screening field defects, loss of vision or evidence of optic neuropathy require formal visual field assessment. Also, employ formal field testing in patients with acute or chronic headache syndromes and transient vision loss despite a normal ophthalmologic examination, as slow-growing intracranial masses and migraine-associated cerebrovascular accidents may be identified.
Frequency doubling technology (FDT) has been developed as a screening tool for glaucoma.11 Advantages include efficiency and high sensitivity and specificity when compared to SAP in assessing field loss from optic neuropathy, with limitations in ascribing field loss located to the chiasm and retrochiasm.12 However, an updated model, FDT (Humphrey Matrix), was found to exhibit greater sensitivity for optic nerve, chiasmal and retrochiasmal disorders when compared to its former counterpart.13,14
Optical Coherence Tomography
Currently, OCT is commonly used to demonstrate pathophysiologic phenomena in a variety of neuro-ophthalmic disorders. It has been studied in several neuro-ophthalmic conditions, including anterior ischemic optic neuropathy, optic neuritis/multiple sclerosis, neuromyelitis optica, idiopathic intracranial hypertension, migraine, optic nerve head drusen, compressive optic neuropathy and Leber’s hereditary optic neuropathy, as well as Alzheimer’s and Parkinson’s disease.15,16
OCT allows the clinician to quantify the thickness of the retinal nerve fiber layer (RNFL), which is useful in managing disorders of the optic nerve and residual effects of intracranial processes. In particular, it can be of great clinical value in differentiating a low-grade disc edema from pseudo-disc edema, namely optic nerve head drusen (ONHD). Specifically, ONHD is associated with a focal, hyperreflective mass on spectral-domain OCT, along with an adjacent hyporeflective region where the outer nuclear layer covers the drusen. Also, ONHD has a much thinner peripapillary RNFL, while disc edema has a much thicker RNFL in the nasal quadrant.17
|
|
|
Neuroimaging
Appropriately diagnosing and managing patients with neuro-ophthalmic disease can be both challenging and rewarding. The nature of the disease course—whether urgent or emergent—dictates which neuroimaging study will deliver the most essential information in a timely manner. The most common clinical indications for the use of neuroimaging are as follows: undetermined visual loss, unilateral or bilateral visual field defects and scotomas, anisocoria, ptosis, proptosis, diplopia, ophthalmoplegia, oscillopsia, optic nerve anomalies and pupillary defects.
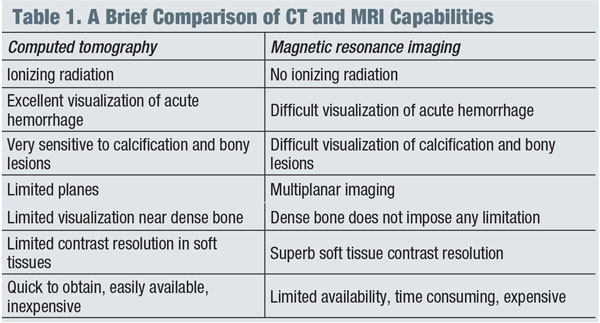
In general, magnetic resonance imaging (MRI) is the modality of choice for imaging suspected neuro-ophthalmic disease processes, while computed tomography (CT) is more appropriate for evaluating intracranial bleeding, osseous lesions of the bony orbit or optic nerve calcifications. (See “A Brief Comparison of CT and MRI Capabilities.")
Additionally, both CT and MR angiography have been successful as non-invasive procedures for studying the arteries in cases of suspected intracranial arterial abnormality.18 These non-invasive angiographic studies may be used with conventional studies to more accurately assess patients with documented or suspected vascular malformations, intracranial aneurysms and neoplastic vascular growths for which conventional MRI/CT is unremarkable or insufficient.
Nonorganic Vision Loss
Patients who describe and present with physical illness in the absence of an organic cause are referred to as having functional loss. In such cases, it is important to take into account the following considerations: nature of the symptoms, attitude and motivation of the individual. Additionally, most nonorganic disorders can be categorized by three types: malingering, Munchhausen syndrome and psychogenic. Patients who present with nonorganic neuro-ophthalmic disorders most commonly complain of dysfunction of vision, visual field, ocular motility, pupil size and reactivity, eyelid position and function, and cornea sensation.
The most common cause of functional illness is vision loss. Individuals generally present with severe vision loss in one or both eyes despite a normal ophthalmologic examination without refractive error. Such cases present a diagnostic challenge and often occupy a great deal of chair time. Following meticulous history, some of the more common in-office tests are SAP, optokinetic (OKN) drum, mirror test, finger touching, stereopsis, high plus lens refraction and the 4 base-out prism test. These tests allow for an object assessment of visual function and binocularity.19
As optometrists, our profession continues to evolve. Managing and comanaging neuro-ophthalmic cases are well within our scope of practice. High-tech office equipment is rarely required for diagnosis and management of these disorders, and most optometric offices are equipped with the necessary resources.
Often, it can be difficult for patients to see a neuro-ophthalmologist, as tertiary practices are fewer in number, appointments are often limited and the distance to travel is usually greater. As a result, optometrists have now become vital providers in serving patients with neuro-ophthalmic disease.
Dr. Trottini is in practice at Outlook Eyecare in Monroe Township, NJ.
Dr. DelGiodice is in practice at Associated Eye Physicians in Clifton, NJ.
1. Cornblath WT, Butter CM, Barnes LL, Hasselbach MM. Spatial characteristics of cerebral polyopia: a case study. Vision Res. 1998 Dec;38(24):3965-78.
2. Athappilly G, Pelak VS, Mandava N, Bennett JL. Ischemic optic neuropathy. Neurol Res. 2008 Oct;30(8):794-800.
3. Hainer BL, Matheson EM. Approach to acute headache in adults. Am Fam Physician. 2013 May 15;87(10):682-7.
4. Ramat S, Leigh RJ, Zee DS, Optican LM. What clinical disorders tell us about the neural control of saccadic eye movements. Brain. 2007 Jan;130(Pt 1):10-35.
5. Trobe JD, Acosta PC, Krischer JP, Trick GL. Confrontation visual field techniques in the detection of anterior visual pathway lesions. Ann Neurol. 1981 Jul;10(1):28-34.
6. Johnson LN, Baloh FG. The accuracy of confrontation visual field test in comparison with automated perimetry. J Natl Med Assoc. 1991 Oct;83(10):895-8.
7. Cibis GW, Tripathi RC, Tripathi BJ. Glaucoma in Sturge-Weber syndrome. Ophthalmology. 1984 Sep;91(9):1061-71.
8. Trobe JD, Glaser JS, Cassady J, et al. Nonglaucomatous excavation of the optic disc. Arch Ophthalmol. 1980 Jun;98(6):1046-50.
9. Rader J, Feuer WJ, Anderson DR. Peripapillary vasoconstriction in the glaucomas and the anterior ischemic optic neuropathies. Am J Ophthalmol. 1994 Jan 15;117(1):72-80.
10. Kedar S, Ghate D, Corbett JJ. Visual fields in neuro-ophthalmology. Indian J Ophthalmol. 2011 Mar-Apr;59(2):103-9.
11. Wall M, Neahring RK, Woodward KR. Sensitivity and specificity of frequency doubling perimetry in neuro-ophthalmic disorders: a comparison with conventional automated perimetry. Invest Ophthalmol Vis Sci. 2002 Apr;43(4):1277-83.
12. Noval S, Contreras I, Rebolleda G, et al. A comparison between Humphrey and frequency doubling perimetry for chiasmal visual field defects. Eur J Ophthalmol. 2005 Nov-Dec;15(6):739-45.
13. Huang CQ, Carolan J, Redline D, et al. Humphrey Matrix perimetry in optic nerve and chiasmal disorders: comparison with Humphrey SITA standard 24-2. Invest Ophthalmol Vis Sci. 2008 Mar;49(3):917-23.
14. Taravati P, Woodward KR, Keltner JL, et al. Sensitivity and specificity of the Humphrey Matrix to detect homonymous hemianopias. Invest Ophthalmol Vis Sci. 2008 Mar;49(3):924-8.
15. Subei AM, Eggenberger ER. Optical coherence tomography: another useful tool in a neuro-ophthalmologists armamentarium. Curr Opin Ophthalmol. 2009 Nov;20(6):462-6.
16. Pasol J. Neuro-ophthalmic disease and optical coherence tomography: glaucoma look-alikes. Curr Opin Ophthalmol. 2011 Mar;22(2):124-32.
17. Johnson LN, Diehl ML, Hamm CW, et al. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009 Jan;127(1):45-9.
18. Weishaupt D, Köchli DV, Marincek B. How Does MRI Work? An Introduction to the Physics and Function of Magnetic Resonance Imaging. Berlin, Germany: Springer-Verlag Berlin and Heidelberg GmbH & Co. K; 2008.
19. Miller NR, Newman NJ, Biousse V, Kerrison JB. Walsh and Hoyt’s Clinical Neuro-Ophthalmology: The Essentials. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2008.

