 |
An 80-year-old white female presented for acute vision loss in her left eye. She reported symptoms of mild fatigue and a recent 10-pound weight loss. While there were no reported allergies, her history was positive for dyslipidemia, BP was 125/80 and she was showing signs of early dementia. She reported that she smokes two cigarettes a day and takes Lipitor.
In the left eye, the patient demonstrated light perception only with pupils showing a grade 4 afferent pupillary defect (APD). Counting fingers were full in the right eye and non-existent in the left. Intraocular pressure was 14mm Hg in each eye and there was no anterior or posterior inflammation found. There were no color plates in the left eye and a posterior pole examination showed pale swelling of the left optic nerve. As these symptoms are consistent with arteritic AION, an immediate neuro-ophthalmology consult was obtained.
Results from subsequent testing demonstrated that her erythrocyte sedimentation rate (ESR) was 100, she had elevated C-reactive protein at 5 and elevated platelets. A temporal artery biopsy showed inflammation and intravenous (IV) methylprednisolone was administered at a dose of 1,000mg daily for three days. From there, she was put on high dose of oral prednisone 80mg/day and he has been treated on steroids for over a year.
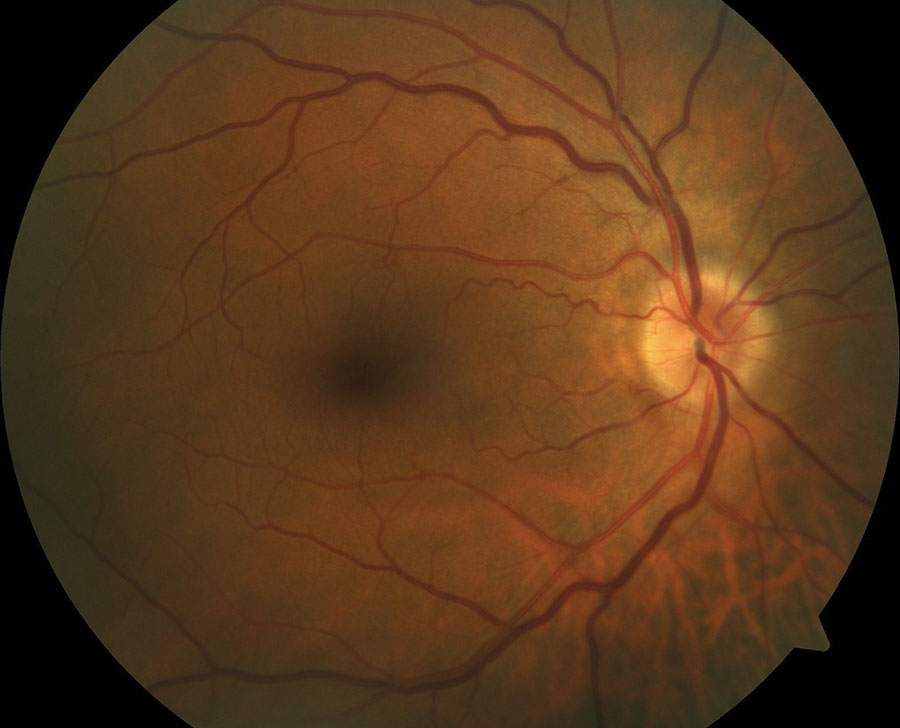 |
| A 60-year-old white male with systemic hypertension and optic nerve typical of a ‘disc at risk.’ A small nerve head with a small c/d ratio is a risk factor for non-arteritic AION, as is his hypertension. |
The Many Sides of ION
Ischemic optic neuropathy (ION) or infarction of the optic nerve can be anterior (AION) or posterior (PION). Both types can be arteritic, non-arteritic or perioperative. Non-arteritic ION, which occurs more frequently and affects adults age 50 and older, tends to cause less severe vision loss than the arteritic variant. Arteritic, on the other hand, affects an older population, i.e., age 70 and above.
As the only common symptom among the variants is painless vision loss, monitoring for the many systemic implications in your patients can help prevent further vision loss in the affected and fellow eye.
Let’s go right to the heart of the matter: the optic nerve. Moving from the anterior to posterior regions of the nerve, are four distinct sections: intraocular (AKA, the optic nerve head or optic disc), intraorbital, intracanalicular and intracranial.
The anterior segment of the optic nerve lies between the optic disc and the site of entry where the central retinal artery enters the nerve. This part is supplied by two vascular networks: the peripheral system and the axial vascular system, present in 75% of optic nerves and supplied by 1–8 intraneural branches of the central retinal artery.1
The posterior segment of the optic nerve, on the other hand, lies between the site of entry of the central retinal artery and the orbital apex, directly prior to entering the intracanalicular portion. This part of the nerve is primarily supplied by the peripheral vascular system and multiple small collateral arteries. These typically stem directly from the ophthalmic artery and less often from other orbital arteries.1,2
Since the intraorbital portion of the nerve is supplied by more than one arterial system, watershed vascular zones exist within the nerve. Within these watershed zones, the intraorbital optic nerve suffers low perfusion pressure, causing areas within the watershed zone to be most vulnerable. Structural abnormalities of the optic nerve, such as a crowded nerve head with a small cup and other vascular risk factors, leave many patients predisposed to the development of AION.
While the development of AION is primarily due to ischemia of the prelaminar and laminar areas where the nerve exits the globe, PION has been linked primarily to ischemia of the intraorbital portion. It’s important to note that this potentially devastating variant can be characterized by the acute, painless vision loss in one or both eyes and can present without optic disc swelling.
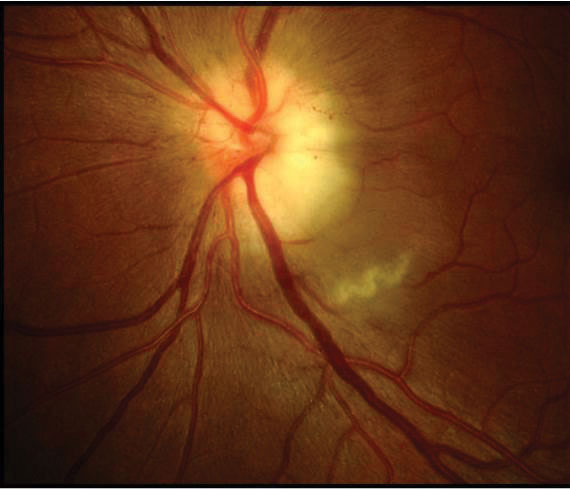 |
| An 80-year old white female with acute loss. VA is light perception in the left eye. Pale, swollen nerve is consistent with arteritic AION (GCA). |
Risk Factors on the Radar
When atherosclerotic narrowing of the posterior ciliary arteries occurs, the eye may be predisposed to non-arteritic AION, particularly after a hypotensive episode.1,3 So, while there are typically no defined medical conditions connected to non-arteritic ION, it’s important to look out for factors contributing to atherosclerosis such as diabetes, smoking, dyslipidemia and hypertension, obstructive sleep apnea, certain drugs (e.g., amiodarone, possibly phosphodiesterase-5 inhibitors) and hypercoagulable disorders.5 Vision loss on awakening leads clinicians to suspect nocturnal hypotension as a potential cause of the non-arteritic AION.4,5
Any of the inflammatory arteritides, especially giant cell arteritis (GCA), can precipitate the arteritic type of infarction.1,3 Acute ischemia in these cases can cause optic nerve edema, which will then further worsen the ischemia.
Making the Diagnosis
Before making a diagnosis, key clinical factors such as the state of the optic disc should be thoroughly investigated. In the presence of AION, the optic disc will be edematous, and the swollen nerve fibers will obscure the fine surface vessels of the nerve. The disc edema may present in a sectoral fashion and hemorrhages may surround the nerve head. The disc may appear pale in the arteritic variety and hyperemic in the non-arteritic type.
In both arteritic and non-arteritic types, perimetry will often demonstrate a defect in the inferior and central fields. Additionally, small and crowded nerve head is a predisposing risk factor for the development of non-arteritic AION, while a large cup-to-disc ratio in the fellow eye should make one think about arteritic AION in the affected eye.3-5 For this reason, when AION is suspected, the clinician should examine the fellow eye to see if it has a “disc at risk,” as it’s called. OCT may be used to further assess the disc edema, ganglion cell thickness and retinal nerve fiber layer thickness, as well as documenting resolution versus stability or progression.
While diagnosis of optic nerve infarction is based mainly on clinical evaluation, ancillary testing may be necessary. Most importantly, the clinician must first rule out the arteritic form, which would require emergency treatment to protect the fellow eye. Immediate tests should include ESR, complete blood count (CBC) and C-reactive protein. ESR is usually dramatically elevated in the arteritic form, often exceeding 100mm/h, and typically normal in the non-arteritic variant. CBC is done to identify thrombocytosis (>400×103/µL), which adds to the positive and negative predictive value of using ESR alone.3-5
If GCA is suspected, a temporal artery biopsy should be performed as soon as possible. Monitoring changes in C-reactive protein level will be necessary for tracking disease activity and the response to treatment. For isolated cases of progressive vision loss, neuroimaging may be obtained to rule out compressive lesions.5
Lastly, narrowing the diagnosis for non-arteritic ION may include testing for obstructive sleep apnea, especially where symptoms such as excessive daytime sleepiness, obesity or loud snoring are present.
Table 1. Common Symptoms and Signs in Patients with ION 3-5
|
Treatment
While up to 40% of eyes with non-arteritic AION spontaneously recover some useful vision, it should be noted that vision loss in arteritic AION, when caused by GCA, will typically be irrevcoverable.5 Arteritic ION, for this reason, must be treated immediately with systemic high-dose steroids to prevent further loss and protect the fellow eye. Inadequate treatment can cause relapses and additional vision loss.
Oral prednisone is the most frequent first-line therapy, though intravenous methylprednisolone has been recommended for severe cases. It is prudent to seek comanagement with neurology or neuro-ophthalmology in these such cases.4,5 For non-arteritic ION, the clinician should investigate for vasculopathic risk factors and sleep apnea and also check blood pressure, glycosylated hemoglobin (A1C) and lipids.5 As always, patients should be encouraged to maintain a healthy diet, exercise, and avoid smoking.
With a watchful eye on the potential symptoms and systemic implications of ischemic optic neuropathy, we as clinicians can make an early diagnosis and initiate the proper treatment plan to prevent further vision loss.
1. Hayreh SS. Anterior ischaemic optic neuropathy I. Terminology and pathogenesis. Br J Ophthalmol 1974; 58:955–963. 2. Hayreh SS. Posterior ischaemic optic neuropathy: clinical features, pathogenesis, and management. Eye 2004; 18:1188–1206. 3. Arnold, Anthony C. “Ischemic optic neuropathy.” Clinical neuro-ophthalmology 1 2005: 349-384. 4. Sohan Singh Hayreh, Ischemic optic neuropathy, In Progress in Retinal and Eye Research 2009; Volume 28, Issue 1, pp. 34-62. 5. Atkins EJ, Bruce BB, Newman NJ, Biousse V. Treatment of Nonarteritic Anterior Ischemic Optic Neuropathy. Survey of ophthalmology. 2010;55(1):47-63. doi:10.1016/j.survophthal.2009.06.008. 6. Sadda SR, Nee M, Miller NR, Biousse V, Newman NJ, Kouzis A. Clinical spectrum of posterior ischemic optic neuropathy. Am J Ophthalmol 2001; 132:743–750. |

