 |
The macula is one of the most important ocular structures and is an essential part of any funduscopic examination. It is located approximately 3mm, or two disc-diameters, temporal to the optic nerve head and houses the fovea at its center. One of its many unique properties is that the macula contains the highest density of rod and cone photoreceptors, resulting in the sharpest visual acuity in this region.1
This delicate area is sensitive to change and can often be difficult to examine. Ancillary testing and a thorough patient history can help distinguish macular diseases from other types of ocular disorders. A simple and often overlooked test that can aid in these situations is the Amsler grid.
 |
|
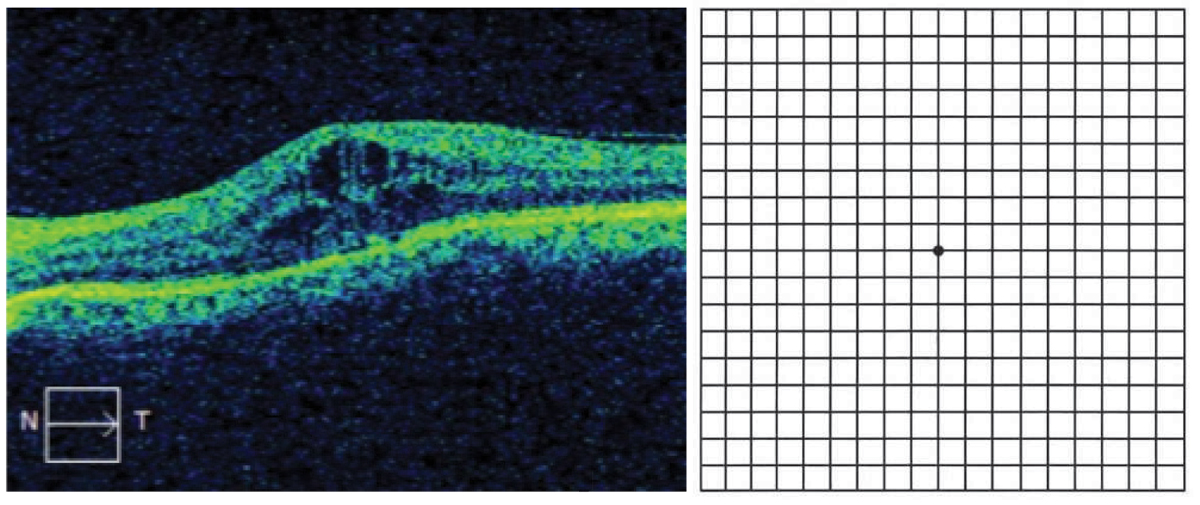
Cystoid macular edema (left), which would manifest as central metamorphopsia on Amsler grid (right). Click image to enlarge. |
Background
Named after Marc Amsler, a Swiss ophthalmologist active in the 1940s, the Amsler grid is a 10cm-by-10cm box that contains a series of smaller squares.1-4 Each square within the grid subtends an angle of approximately 1°.4 When viewed centrally at a testing distance of 33cm, it maps the central 10° field corresponding to the macula.1
Amsler grid testing is typically performed monocularly with best near vision correction in place and undilated with adequate and uniform illumination over the grid. The patient is then asked to fixate on the central dot and should simultaneously be able to view all four corners of the grid. Failure to do so could indicate peripheral field loss of a non-macular origin.
Afterwards, ask the patient to note if any areas or boxes within the grid appear wavy, distorted, dim or broken. If so, it is a good idea to have the patient draw the abnormality, both to help identify the pathological area as it corresponds to the macula and to monitor the status or progression of the defect over time.1
A positive test indicates that the patient is experiencing either metamorphopsia or a scotoma, both of which indicate an underlying macular disease origin. Metamorphopsia is the distortion of an object, whether that be in size, shape or other kind of appearance. In the initial manifestation of metamorphopsia, patients may perceive an “instability” in their vision, rather than true distortion.1
There are two variations of metamorphopsia: micropsia and macropsia. In micropsia the parallel lines in the grid appear closer together, whereas in macropsia the lines are distorted further and curved away from each other. Micropsia is more common, and patients may perceive that an object or image is smaller than it appears vs. larger in macropsia. It has been suggested that when retinal elements are displaced in closer proximity to one another, this manifests as macropsia. In a similar manner, a condition such as macular edema where fluid elevates and displaces the photoreceptors away from each other would cause micropsia.1,4
Recent evidence suggests metamorphopsia is due to a combination of cortical and retinal changes. Originally, it was thought that metamorphopsia arose purely from the structural changes that occur within the outer retinal layers—mainly photoreceptors—affected in macular disease, ultimately leading to impaired light signal transduction. This is evident in cases such as cystoid macular edema. Recent literature, however, suggests that inner retinal changes and cortical processing are both contributory to the phenomenon.
In cases of epiretinal membrane formation, thickening of the inner retinal layers correlates highly with metamorphopsia. This is possibly due to the presence of Müller, amacrine, horizontal and bipolar cells within these layers that are disrupted, leading to impaired synaptic function within the photoreceptors. In cases of persistent maculopathy or following treatment of neovascular age-related macular degeneration (AMD), metamorphopsia is not only caused by displacement of light onto the retina but also by changes in cortical processing.4
 |
|
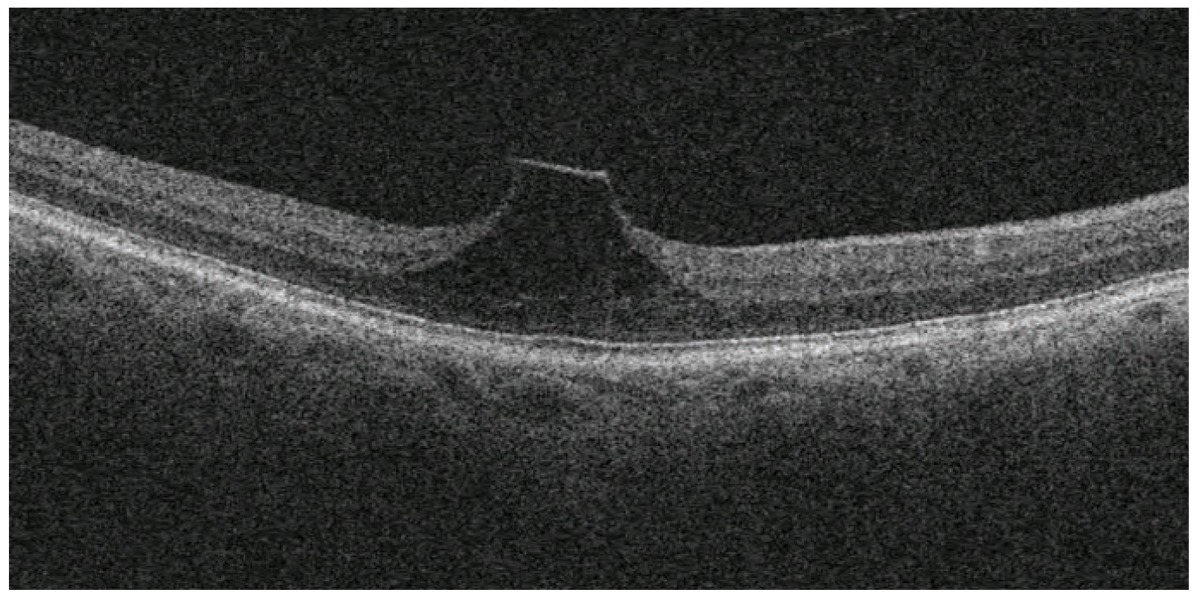
This patient has vitreomacular traction and is monitoring symptoms of metamorphopsia with at-home Amsler grid testing. Click image to enlarge. |
A scotoma is the absence of a spot on the visual field, which can either be absolute or relative. In an absolute scotoma, the missing area is present regardless of any variation in stimuli. In contrast, relative scotomas in the visual field can become more visible with increases in stimuli. The Amsler grid test is more useful in identifying absolute scotomas, where a patient perceives a broken box or missing line. An underlying macular condition that would yield this type of result is a macular hole.1
Pros and Cons
Amsler grid testing offers several advantages. Its chief utility is that it is cost-effective and can be efficiently administered chairside. It is also readily available and can be sent home with the patient for self-monitoring. This testing does not require a great deal of patient training due to its ease of use.2 Oftentimes, a patient may subjectively report metamorphopsia in the setting of absent or very subtle funduscopic signs when standard visual acuity measurements are normal.4 In such cases, an abnormality on Amsler grid testing would indicate need for a more careful examination or follow-up.
It is well documented that the Amsler grid is optimal for ocular diseases confined to the macula. These conditions include, but are not limited to, wet or neovascular AMD, central serous chorioretinopathy, epiretinal membranes, cystoid macular edema and hydroxychloroquine-associated macular toxicity.1
Also of note, this test can be effective in detecting field defects secondary to glaucoma. In a study investigating the efficacy of Amsler grid testing among patients with advanced glaucoma in Ethiopia, the black-on-white grid resulted in a sensitivity of 80.4% and a specificity of 95.4%. The study concluded that the Amsler grid can be used as an effective screening tool in areas where a Humphrey visual field perimeter is not readily available and in the detection of glaucomatous visual field defects that affect the macula.2
While this test offers many advantages, it lacks the ability to properly test patients with loss of central fixation commonly documented in those with macular disease, which particularly affects the fovea.3 Patients with significant central loss have a difficult time with test administration due to their inability to locate the central fixation point.
Additionally, a compensatory “filling in” mechanism that is a normal function of visual perception can alter test results. This is called the cortical completion phenomenon. A common example of this phenomenon is the eye’s blind spot. Even in a normal eye, there is an absence of photoreceptors in this unique anatomical region. However, we do not perceive this area as “blind” or absent in our visual field because of a cortical mechanism that fills this area with a similar appearance to its surrounding environment. As a result, our perception is that it is not absent visual space.5 In this manner, a patient undergoing Amsler testing can have a similar compensatory mechanism and be unable to distinguish a small scotoma from the surrounding area, making the test results of little to no clinical value.
Takeaways
As with all clinical tests, the Amsler grid offers a unique variety of benefits that aid in the diagnosis and management of ocular disease, particularly retinal disorders. However, it is not without its disadvantages. As such, it should be used in conjunction with a full history and ophthalmic examination. Understanding the test procedure, anatomical correlations and interpretation of results is necessary in using the Amsler grid to its fullest potential.
Dr. Labib graduated from Pennsylvania College of Optometry, where she now works as an associate professor. She completed her residency in primary care/ocular disease and is a fellow of the American Academy of Optometry and a diplomate in the Comprehensive Eye Care section. She has no financial interests to disclose.
1. Tripathy K, Salini B. Amsler grid. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. 2. Gessesse GW, Tamrat L, Damji KF. Amsler grid test for detection of advanced glaucoma in Ethiopia. PLoS One. 2020;15(3):e0230017. 3. Isaac DL, Avila MP, Cialdini AP. Comparison of the original Amsler grid with the preferential hyperacuity perimeter for detecting choroidal neovascularization in age-related macular degeneration. Arq Bras Oftalmol. 2007;70(5):771-6. 4. Midena E, Vujosevic S. Metamorphopsia: an overlooked visual symptom. Ophthalmic Res. 2015;55(1):26-36. 5. Pessoa L, De Weerd P, eds. Filling-In: From Perceptual Completion to Cortical Reorganization. Oxford University Press; 2003. |

